��Ŀ����
����Ŀ��̼����(SrCO3)�����������ɫ���桢ӫ�ⲣ���ȡ�С��ͬѧΪ�ⶨijSrCO3��NaCl����������SrCO3�������������������ձ��н������ʵ�飬ʵ������ϡ��������ʵ�����������ͬ�������������±���ʾ��
�ձ���� | �� | �� | �� |
ϡ��������/g | 100 | 200 | 300 |
������������/g | 100 | 100 | 100 |
��Ӧ���ձ������ʵ�����/g | 195.6 | 291.2 | 391.2 |
����
(1)�ձ����в���CO2������Ϊ____g��
(2)ԭ����������SrCO3����������____(��������ȷ��0.1%)��
(��ʾ��SrCO3+2HCl===SrCl2+CO2��+H2O)
���𰸡�8.8 29.6%
��������
��1�����ݼ���200g�����������Ϊ200g+100g-291.2g=8.8g��������300g��������Ҳ��300g+100g-391.2g=8.8g��˵���ڶ���ʵ���й����Ѿ���ȫ��Ӧ��
�裺ԭ����������SrCO3������Ϊx��
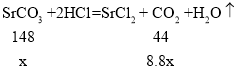
![]() x=29.6g
x=29.6g
�������SrCO3����������=![]() ��100%=29.6%
��100%=29.6%
��ԭ����������SrCO3����������Ϊ29.6%��

��ϰ��ϵ�д�
�����Ŀ