��Ŀ����
(6��) ij��һ������Ũ����Ĺ�����������25t������������Ϊ98%��Ũ����й©������·���������ӣ��ӵ������������ٱ�������������ʯ�ҽ�����Ҫ�ɷ�Ϊ�������ƣ��к������������顣��ش�
��1��25t������������Ϊ98%��Ũ�����к�H2SO4������Ϊ ����2�֣�
��2�����㣺�к�й©�����ᣬ��������Ҫ���ٶ��������ƣ���д��������̣�2�֣�
��3������һ���������ᣬ�����ϼȿ���m1�ֵ��������Ʒ�ĩ��Ҳ��ѡ��m2�ֵ������Ʒ�ĩ��������m3��̼��Ʒ�ĩ����m1��m2��m3����ֵ��С��ϵΪ ����2�֣�
��1��25t������������Ϊ98%��Ũ�����к�H2SO4������Ϊ ����2�֣�
��2�����㣺�к�й©�����ᣬ��������Ҫ���ٶ��������ƣ���д��������̣�2�֣�
��3������һ���������ᣬ�����ϼȿ���m1�ֵ��������Ʒ�ĩ��Ҳ��ѡ��m2�ֵ������Ʒ�ĩ��������m3��̼��Ʒ�ĩ����m1��m2��m3����ֵ��С��ϵΪ ����2�֣�
��1�� 24.5t ��2��18.5t ��3�� m3��m1��m2
��1�����ʵ�����=��Һ��������������������=25t��98%=24.5t��
��2�����û�ѧ����ʽ����ɵó��������Ƶ�������
�⣺����������Ҫ�������Ƶ�����Ϊx
H2SO4 + Ca(OH)2 ="==" CaSO4 + 2H2O
98 74
24.5t x
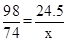 x=18.5t
x=18.5t
��3�����û�ѧ����ʽ�ֱ�����m1��m2��m3����ֵ��Ȼ��Ƚϴ�С��ϵ��
H2SO4+ Ca(OH)2 ="==" CaSO4 + 2H2O H2SO4+ CaO="==" CaSO4 + H2O
98 74 98 56
24.5t m1 24.5t m2
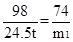
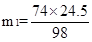
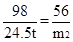
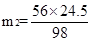
H2SO4+ CaCO3 ="==" CaSO4 + H2O + CO2��
98 100
24.5t m3
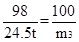

�ʣ�m3��m1��m2
��2�����û�ѧ����ʽ����ɵó��������Ƶ�������
�⣺����������Ҫ�������Ƶ�����Ϊx
H2SO4 + Ca(OH)2 ="==" CaSO4 + 2H2O
98 74
24.5t x
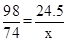 x=18.5t
x=18.5t��3�����û�ѧ����ʽ�ֱ�����m1��m2��m3����ֵ��Ȼ��Ƚϴ�С��ϵ��
H2SO4+ Ca(OH)2 ="==" CaSO4 + 2H2O H2SO4+ CaO="==" CaSO4 + H2O
98 74 98 56
24.5t m1 24.5t m2
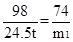
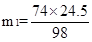
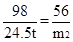
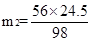
H2SO4+ CaCO3 ="==" CaSO4 + H2O + CO2��
98 100
24.5t m3
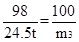

�ʣ�m3��m1��m2

��ϰ��ϵ�д�
�����Ŀ
