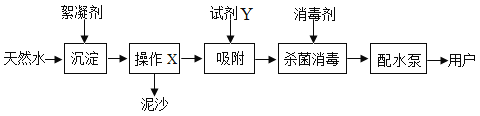
��Ŀ����
����Ŀ�������ʵĺ�ۡ��ۺͷ���֮�佨����ϵ�ǻ�ѧѧ�Ƶ��ص㡣����ͼʾ�ش��������⣺
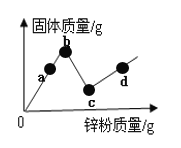
��1�����ʵ���ɺ�����ͼ��ʾ��ͼ������ʾ����___________��
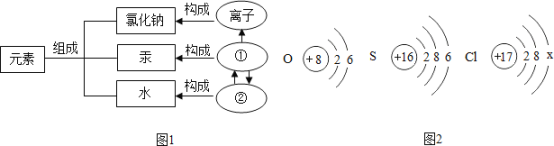
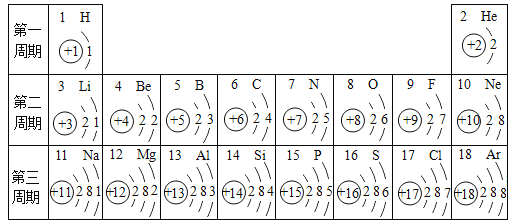
��2��ͼ 2 ��������������Ԫ�ص�ԭ�ӽṹʾ��ͼ��
�� ��ԭ�ӵĽṹʾ��ͼ�� x ����ֵ��___________��
�� ����������Ԫ�صĻ�ѧ���ʾ��������Ե�ԭ��������ԭ�ӵ�___________��ͬ��
�� �������������ˮ����Ϊ���ᣬ����������������������������������Һ��ϡ�����ֻ�ϣ�������Һ���������ʲ���Ӧ���� ___________(����ĸ)��
A CuSO4B Fe C BaCl2D SO2
��ϡ���ᡢϡ����������ƵĻ�ѧ���ʣ�����Ϊ��Һ�ж�������ͬ��____________�������ӷ��ţ���
���𰸡�ԭ�� 7 ���������� B H+
��������
��1�������ɹ�ԭ�ӹ��ɵģ���ͼ�Тٱ�ʾ����ԭ�ӣ�
��2����ԭ����������=���������������ԭ�ӵĽṹʾ��ͼ�� x ����ֵ��7��
������������Ԫ�صĻ�ѧ���ʾ��������Ե�ԭ��������ԭ�ӵ�������������ͬ��
�۸��ݷ�Ӧ��![]() �����Կ����������������ᷴӦʱ������Ҫ������������ȡ���������������ͬ������������Һ��ϡ������ʱ�������ƻ���ʣ�࣬���Ի�Ϻ����Һ�л���NaOH��
�����Կ����������������ᷴӦʱ������Ҫ������������ȡ���������������ͬ������������Һ��ϡ������ʱ�������ƻ���ʣ�࣬���Ի�Ϻ����Һ�л���NaOH��![]() ��
��
A��CuSO4���������Ʒ�Ӧ����������ͭ�����������ƣ�
B��Fe���������ơ��Ȼ��ƾ�����Ӧ��
C��BaCl2�������Ʒ�Ӧ�����Ȼ��ƺ����ᱵ������
D��SO2������������Һ��Ӧ�����������ƺ�ˮ��
���B��
��ϡ���ᡢϡ����������ƵĻ�ѧ���ʣ�����Ϊ��Һ�ж�������ͬ��H+��

 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�