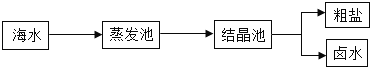
��Ŀ����
����Ŀ��Ϊ�ⶨij����������淋Ĵ��ȣ��������������Ƿ�����ͼ��ǩ�����С��ȡ15g�û�����Ʒ�����Һ����Ʒ�õ����ʶ�����ˮ�����뵽100g�Ȼ�����Һ�У������ɷֲ����Ȼ�����Һ������ѧ��Ӧ��������ǡ����ȫ��Ӧ�����ˡ�ϴ�ӡ���ɣ��õ�23.3g������һ�������IJ�������Һ���ش��������⣺

��1���ڱ�ǩ��д������淋Ļ�ѧʽ��
��2����ͨ������ȷ���û���������淋Ĵ����Ƿ����ǩ�����
��3��������Һ�м�����ٿ�ˮ�������Ƴ�3%���Ȼ��Ӫ��Һ��
���𰸡���1����NH4��2SO4��2�����ǩ��ע����
��3��������Һ�м���264.97gˮ��
����������1������淋Ļ�ѧʽ�ǣ�NH4��2SO4����ע����ͼ��ʾ��

��2�������������Ϊx�������Ȼ������Ϊy��
��NH4��2SO4+BaCl2�TBaSO4��+2NH4Cl��
132 233 107
x 23.3g y
![]() =
=![]() =
=![]() ��
��
x=13.2g��y=10.7g��
���������������![]() ��100%=88%��
��100%=88%��
���ǩ��ע������
��3�������ˮ������Ϊz��
����������![]() ��100%=3%��
��100%=3%��
z=264.97g��
��������Һ�м���264.97gˮ�������Ƴ�3%���Ȼ��Ӫ��Һ��

��ϰ��ϵ�д�
 ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�
�����Ŀ