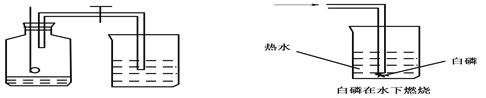��Ŀ����
����Ŀ��ij����С�鰴��ͼ��ʾװ����ȡ������̼�������������̼�����ʣ�����װ��B��֤��������̼��ˮ��Ӧ����̼�ᣬB�г�������ˮ�⣬��Ӧ������Լ��ǣ������������ܿ�����ʱ��B�п�����ʵ�������ǣ�________���䷴Ӧ�Ļ�ѧ����ʽΪ��________��
�����ܶϿ�һ��ʱ�����B�е�������________���䷴Ӧ�Ļ�ѧ����ʽΪ________��
Ϊ�ⶨ����ʯ��ʯ��̼���Ƶ������������ÿ���С��ȡ��һЩ��ʯ����ȡϡ����200g������ƽ���ֳ�4�ݣ�����ʵ�飬�������£�
ʵ�� | ��1�� | ��2�� | ��3�� | ��4�� |
������Ʒ������/g | 5 | 10 | 15 | 20 |
����CO2������/g | 1.54 | 3.08 | 4.4 | m |
���ݱ���������������㣺
��1���ļ��Ӧ��������ʣ�ࡣ
��2���ϱ���m����ֵ�ǡ�
��3���Լ�������ʯ��ʯ��̼��Ƶ�����������
���𰸡�ʯ����Һ��ʯ����Һ����ɫ���ɫCO2+H2O==H2CO3����ɫ����ʧ�ֱ���ɫ��H2CO3==H2O+CO2��
��1����һ�����ݣ�2��4.4��3��70��
��������
���������������ѧ֪ʶ��������Ϣ֪������װ��B��֤��������̼��ˮ��Ӧ����̼�ᣬB�г�������ˮ�⣬��Ӧ������Լ���ʯ����Һ�������������ܿ�����ʱ��B�п�����ʵ�������ǣ�ʯ����Һ����ɫ���ɫ���䷴Ӧ�Ļ�ѧ����ʽΪ��CO2+H2O==H2CO3�����ܶϿ�һ��ʱ�����B�е���������ɫ����ʧ�ֱ���ɫ���䷴Ӧ�Ļ�ѧ����ʽΪ��H2CO3==H2O+CO2�������ݱ���������������㣺��1���ļ��Ӧ��������ʣ�ࡣ��һ�����ݡ���2���ϱ���m����ֵ��4.4��
CaCO3+2HCl=CaCl2+ H2O+CO2��
100 44
x 1.54g
���x=3.5g
3.5g/5g*100%=70%
��������ʯ��ʯ��̼��Ƶ���������70����
������������ȡ������̼�������������̼�����ʼ����ݻ�ѧ����ʽ���м��㡣

 ��һ������Ԫͬ�����ؾ�ϵ�д�
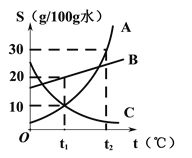
��һ������Ԫͬ�����ؾ�ϵ�д�����Ŀ���±��г��˹�������A�ڲ�ͬ�¶�ʱ���ܽ�ȣ�
�¶ȣ��� | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 |
�ܽ�ȣ�g | 36 | 35 | 34 | 33 | 32 | 31 | 30 | 29 | 28 | 27 |
(1)70��ʱ����ʢ��100gˮ���ձ��м���30g����A������ܽ��γɵ���______________(����͡������͡�)��Һ���ٽ��ձ��������¶Ƚ���20�棬��ʱ��Һ���������ܼ���������Ϊ_____________(�����������)��
(2)ͨ�����ϱ����ݵķ���������A���ܽ������Ӧ����ͼ�е�___________(��ס����ҡ�)��
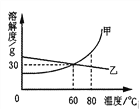
(3)80��ʱ������һ����A���ʵ���Һ�����併�µ�60�棬�Ƿ��й�������?____________(��С��� ��û�С���ȷ����)��