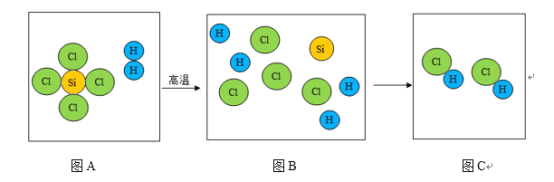
��Ŀ����
����Ŀ�����Ƹ��н����п���ѧʵ�鿼�飬���������������⣺�ٴ����ᴿ���ڶ�����̼����ȡ���ռ�������������ȡ���ռ�������涨��ѧ����ǩȷ�����⣬��ͬѧ��ǩ���ʦ��������������������ҩƷ��ʵ��̨ǰ��
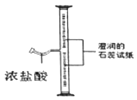
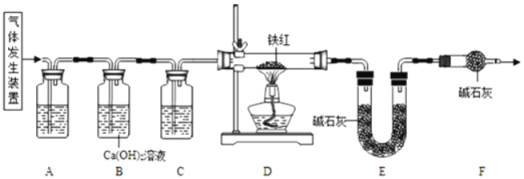
��(1)��ͼ������A��������_____����ͬѧ�鵽�Ŀ�����_____(�����)��ʵ��ǰ��ͬѧ��������ȱ����һ��ҩƷ����ҩƷ��_____��д����ʵ�鷴Ӧ�Ļ�ѧ����ʽ_____��
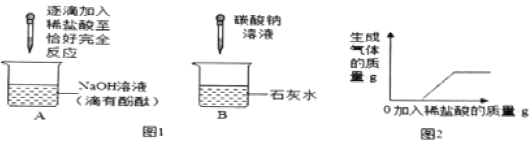
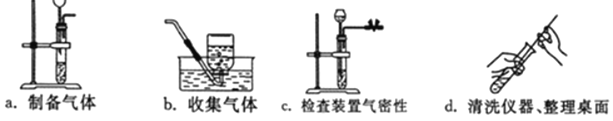
(2)��ͼ�Ǽ�ͬѧʵ��ʱ����Ҫ���裬��Щ�������ȷ˳����___(����ĸ���)������װ���������___(����ĸ���)�������ķ�����_____��
(3)����ѡҩƷ��Ҳ�������һ������������Ʊ�����Ӧ�Ļ�ѧ����ʽΪ_____��
����ͬѧ��ǩ�����ʵ����ʦҪ���ش��������⣬��������ش�
(4)�ڹ��˲����У���������������_____��
���𰸡�����̨ �� �������� 2H2O2![]() 2H2O+O2�� cabd a ������©����û��Һ������ CaCO3+2HCl��CaCl2+H2O+CO2�� ����
2H2O+O2�� cabd a ������©����û��Һ������ CaCO3+2HCl��CaCl2+H2O+CO2�� ����
��������
(1)ͼ1������A������������̨����ͼʾ������ҩƷ��֪����ͬѧ��ȡ������������ȡ���ռ������������ڶ������̵Ĵ�����������ˮ����������ȱ�ٶ������̣��������̨���ۣ��������̣�2H2O2![]() 2H2O+O2����
2H2O+O2����
(2)ͼ2�Ǽ�ͬѧʵ��ʱ����Ҫ���裬��Щ�������ȷ˳����cabd������a���д������ķ����ǽ�����©����û��Һ�����£����cabd��a��������©����û��Һ�����£�
(3)��������ΪҺ�塢��������Ϊ�����ҷ�Ӧ������ȣ����Ը��ݶ�����̼�ķ���װ��Ϊ��Һ��ϲ����ȵ�װ�ã��õ���������̼��ƺ�ϡ�����Ʊ�������̼���䷴Ӧ��ѧ����ʽ��ʾΪ��CaCO3+2HCl��CaCl2+H2O+CO2�������CaCO3+2HCl��CaCl2+H2O+CO2����
(4)�ڹ��˲����У������������������������������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��ѧϰ�������Ļ�ѧ���ʺ���ȤС���ͬѧ����ʵ���ҵ�һƿ����������Һ��������̽����
̽����������⣩ ������������Һ�Ƿ��Ѿ����ʣ�
��������¶���ڿ����б��ʵķ�Ӧ����ʽ_________��___(������������������)���ֽⷴӦ��
��ʵ��̽����
ʵ����� | ʵ������ | ʵ����� |
����1��ȡ�����μ�������______�� | ��������� | ����������Һû�б��� |
����2��ȡ�����μ�����������Һ |
��ʵ����ۣ�����������Һû�б��ʡ�
̽����������⣩ �кͷ�Ӧ�������Ƿ��������仯��
��ʵ��̽����
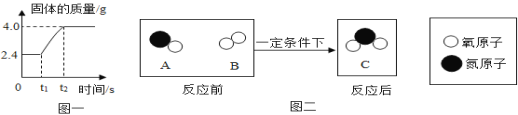
��ͬѧ��ȡ����������������Һ���μ�1~2�η�̪������Һ�в����¶ȼƣ�����μ���ϡ���ᣬ�ߵμӱ߽��裬����¶ȱ仯�����ͼ��ʾ��
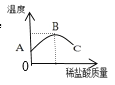
��д���÷�Ӧ�Ļ�ѧ����ʽ___________��ʵ���н����Ŀ����____________��
�ڴ�ͼ���֪���кͷ�Ӧ��____���̣������BC�εij���__________��
��ͬѧ��ȡ�����������ƹ�����ϡ���ᷴӦ����ʵ�飬�ó���ͬ���Ľ��ۡ�С��ͬѧ��Ϊ����ͬѧ�Ľ��۲����ţ�����Ϊ����������___________��