��Ŀ����
�������Ϻ�ˮ�����������������Ź㷺Ӧ�ã�
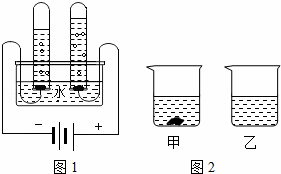
��1����ͼ1��ˮ����ɲⶨ��ʵ�飬�˷�Ӧ�ķ���ʽΪ______��
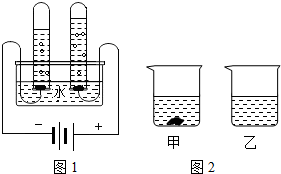
��2��ˮ�Ǻܺõ��ܼ����±���KNO3��KCl�ڲ�ͬ�¶�ʱ���ܽ�ȣ���ش��������⣺
| �¶�/�� | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | |
| �ܽ��/g | KNO3 | 13.3 | 20.9 | 31.6 | 45.8 | 63.9 | 85.5 | 110 | 138 |
| KCl | 27.6 | 31.0 | 34.0 | 37.0 | 40.0 | 42.6 | 45.5 | 48.3 | |
�ڲ�������һ�ֲ����������������ձ��е�ʣ�����ȫ���ܽ⣬������Һ��Ϊ��������Һ������˵������ȷ����______������ĸ����
A���ܼ��������ܲ��䡡�������������� B����Һ�����ʵ���������һ������
C����Һ����һ������������������ D���ɲ������»��ˮ�ķ���
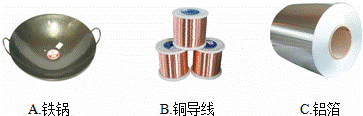
��3������ͼ��֪�������е����Ե���______��

��4������Ʒ�ڿ����лᷢ����ʴ����ԭ������������е�______��ͬ���õĽ����Ϊ��ֹ������Ʒ����ʴ�������е������Ͳ˵�ͨ�������ķ�����______��Ҫ��һ�ַ������ɣ���
��5����̽�������Ļ�ѧ����ʱ��ijͬѧ��������������ϡ�����У��۲쵽��������ʧ����Һ����ɫ��ɻ�ɫ���������ݲ������йط�Ӧ�Ļ�ѧ����ʽΪ______��______��
��6��С������FeSO4��CuSO4�������ʵ���Һ�У�����һ������þ�ۣ�����ַ�Ӧ����Һ��dz��ɫ�����ݴ����������������жϣ�����Ϊ��ȷ����______����д��ţ���
A����ֽ��һ����Cu��������Fe��һ��û��Mg
B����ֽ��һ����Fe��������Mg��Cu
C����Һ��һ����FeSO4��������MgSO4��CuSO4
D����Һ��һ����MgSO4��FeSO4��һ��û��CuSO4��
 2H2��+O2����
2H2��+O2������2�������ܽ�ȿ�֪����40��ʱ��KNO3���ܽ����63.9g��KCl���ܽ����40.0g�������ձ�������ȫ���ܽ��ˣ�˵�������ձ��е�������KNO3��
��A���������¶�ʱ���ձ��е�ʣ�����ȫ���ܽ⣬��Һ��Ϊ��������Һ���ܼ��������䣮��A˵����ȷ��
B���������ܼ�ʱ���ձ��е�ʣ�����ȫ���ܽ⣬��Һ��Ϊ��������Һ����Һ�����ʵ�����������һ������B˵����ȷ��
C�����ʵ����������ˣ���Һ����һ������C˵����ȷ��
D���ɲ������»��ˮ�ķ�����������ʹ�ձ��е�ʣ�����ȫ���ܽ⣬��Һ��Ϊ��������Һ����D˵����ȷ��
��3�������������ˡ����������������Ĵ����ԣ�
��4����Ʒ�ڿ����лᷢ����ʴ����ԭ������������е�������ˮ��ͬ���õĽ����Ϊ��ֹ������Ʒ����ʴ�������е������Ͳ˵�ͨ�������ķ����Dz��ɻ�Ϳ�ͣ�
��5����������������ϡ�����У��۲쵽��������ʧ����Һ����ɫ��ɻ�ɫ���������ݲ������йط�Ӧ�Ļ�ѧ����ʽΪFe2O3+6HCl=2FeCl3+3H2O��Fe+2HCl=FeCl2+H2����
��6�����ڽ����Ļ���Ǵ�С��þ������ͭ������FeSO4��CuSO4�������ʵ���Һ�У�����һ������þ�ۣ�þ����������ͭ��Ӧ��������ͭ��Ӧ��ɺ���������������Ӧ��������Һ��dz��ɫ��˵����þȫ���μ��˷�Ӧ���ɴ˿�֪��
A����ֽ��һ����Cu��������Fe��һ��û��Mg����A˵����ȷ��
B����ֽ�ϲ�һ����Fe��һ��û��Mg��һ����ͭCu����B˵������
C����Һ��һ����MgSO4����C˵������
D����Һ��һ����MgSO4��FeSO4��һ��û��CuSO4����D˵����ȷ��
�ʴ�Ϊ����1��2H2O
 2H2��+O2������2����KNO3����ACD����3��A����4��������ˮ�����ɻ�Ϳ�ͣ���5��Fe2O3+6HCl=2FeCl3+3H2O��Fe+2HCl=FeCl2+H2������6��AD��
2H2��+O2������2����KNO3����ACD����3��A����4��������ˮ�����ɻ�Ϳ�ͣ���5��Fe2O3+6HCl=2FeCl3+3H2O��Fe+2HCl=FeCl2+H2������6��AD����������1������ͼ1�����ķ�Ӧ��д����Ӧ�ķ���ʽ��
��2���ٸ�����40��ʱ��KNO3��KCl�ܽ�ȵĴ�С������
�ڸ��ݱ�����Һ�벻������Һ֮���ת����ϵ������
��3�����ʵ����ʾ������ʵ���;�����ʵ���;��ӳ���ʵ����ʣ�
��4�����������������������ֹ����Ĵ�ʩ��
��5����������������������ķ�Ӧд����Ӧ�ķ���ʽ��
��6�����ݽ������˳��λ����ǰ�Ľ����ܽ�����Ľ�����������Һ���û�����������
����������Ƚ�ȫ�濼���˽������Ϻ�ˮ��֪ʶ���������Ϻ�ˮ�������������������Ź㷺Ӧ�ã�Ӧ��ǿ�й�֪ʶ��ѧϰ��

�������Ϻ�ˮ�����������������Ź㷺Ӧ�á�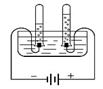
��1������ͼ��ˮ����ɲⶨ��ʵ�飬�˷�Ӧ�ķ���ʽΪ ��
��2��ˮ�Ǻܺõ��ܼ����±���KNO3��KCl�ڲ�ͬ�¶�ʱ���ܽ��,��ش��������⣺
| �¶�/�� | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | |
| �ܽ��/g | KNO3 | 13.3 | 20.9 | 31.6 | 45.8 | 63.9 | 85.5 | 110 | 138 |
| KCl | 27.6 | 31.0 | 34.0 | 37.0 | 40.0 | 42.6 | 45.5 | 48.3 | |
�ڲ�������һ�ֲ����������������ձ��е�ʣ�����ȫ���ܽ⣬������Һ��Ϊ��������Һ������˵������ȷ���� ������ĸ����
A.�ܼ��������ܲ��� B.��Һ�����ʵ���������һ������
C.��Һ����һ������ D.�ɲ������»��ˮ�ķ���
��3������ͼ��֪�������е����Ե��� ��

A.���� B.ͭ���� C.����
��4������Ʒ�ڿ����лᷢ����ʴ����ԭ������������е� ��ͬ���õĽ����Ϊ��ֹ������Ʒ����ʴ�������е������Ͳ˵�ͨ�������ķ����� ��Ҫ��һ�ַ������ɣ���
��5����̽�������Ļ�ѧ����ʱ��ijͬѧ��������������ϡ�����У��۲쵽��������ʧ����Һ����ɫ��ɻ�ɫ���������ݲ������йط�Ӧ�Ļ�ѧ����ʽΪ �� ��
��6��С������FeSO4��CuSO4�������ʵ���Һ�У�����һ������þ�ۣ�����ַ�Ӧ����Һ��dz��ɫ�����ݴ����������������жϣ�����Ϊ��ȷ���� ����д��ţ���
A.��ֽ��һ����Cu��������Fe��һ��û��Mg
B.��ֽ��һ����Fe��������Mg��Cu
C.��Һ��һ����FeSO4��������MgSO4��CuSO4
D.��Һ��һ����MgSO4��FeSO4��һ��û��CuSO4
�������Ϻ�ˮ�����������������Ź㷺Ӧ�á�
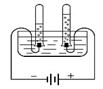
��1������ͼ��ˮ����ɲⶨ��ʵ�飬�˷�Ӧ�ķ���ʽΪ ��
��2��ˮ�Ǻܺõ��ܼ����±���KNO3��KCl�ڲ�ͬ�¶�ʱ���ܽ��,��ش��������⣺
|
�¶�/�� |
0 |
10 |
20 |
30 |
40 |
50 |
60 |
70 |
|
|
�ܽ��/g |
KNO3 |
13.3 |
20.9 |
31.6 |
45.8 |
63.9 |
85.5 |
110 |
138 |
|
KCl |
27.6 |
31.0 |
34.0 |
37.0 |
40.0 |
42.6 |
45.5 |
48.3 |
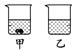
��40 ��ʱ���������ֱ�ʢ��45 g KNO3��KCl������ձ��У�������100g��ˮ������ܽ�������ͼ��ʾ�������ձ��е������� ��
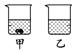
�ڲ�������һ�ֲ����������������ձ��е�ʣ�����ȫ���ܽ⣬������Һ��Ϊ��������Һ������˵������ȷ���� ������ĸ����
A.�ܼ��������ܲ��� B.��Һ�����ʵ���������һ������
C.��Һ����һ������ D.�ɲ������»��ˮ�ķ���
��3������ͼ��֪�������е����Ե��� ��

A.���� B.ͭ���� C.����
��4������Ʒ�ڿ����лᷢ����ʴ����ԭ������������е� ��ͬ���õĽ����Ϊ��ֹ������Ʒ����ʴ�������е������Ͳ˵�ͨ�������ķ����� ��Ҫ��һ�ַ������ɣ���
��5����̽�������Ļ�ѧ����ʱ��ijͬѧ��������������ϡ�����У��۲쵽��������ʧ����Һ����ɫ��ɻ�ɫ���������ݲ������йط�Ӧ�Ļ�ѧ����ʽΪ �� ��
��6��С������FeSO4��CuSO4�������ʵ���Һ�У�����һ������þ�ۣ�����ַ�Ӧ����Һ��dz��ɫ�����ݴ����������������жϣ�����Ϊ��ȷ���� ����д��ţ���
A.��ֽ��һ����Cu��������Fe��һ��û��Mg
B.��ֽ��һ����Fe��������Mg��Cu
C.��Һ��һ����FeSO4��������MgSO4��CuSO4
D.��Һ��һ����MgSO4��FeSO4��һ��û��CuSO4
�������Ϻ�ˮ�����������������Ź㷺Ӧ�ã�
��1����ͼ1��ˮ����ɲⶨ��ʵ�飬�˷�Ӧ�ķ���ʽΪ������
��2��ˮ�Ǻܺõ��ܼ����±���KNO3��KCl�ڲ�ͬ�¶�ʱ���ܽ�ȣ���ش��������⣺
| �¶�/�� | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 |
|
| �ܽ��/g | KNO3 | 13.3 | 20.9 | 31.6 | 45.8 | 63.9 | 85.5 | 110 | 138 |
| KCl | 27.6 | 31.0 | 34.0 | 37.0 | 40.0 | 42.6 | 45.5 | 48.3 |
��40��ʱ���������ֱ�ʢ��45g KNO3��KCl������ձ��У�������100g��ˮ������ܽ�����ͼ2��ʾ�������ձ��е�������������
�ڲ�������һ�ֲ����������������ձ��е�ʣ�����ȫ���ܽ⣬������Һ��Ϊ��������Һ������˵������ȷ��������������ĸ����
A���ܼ��������ܲ��� B����Һ�����ʵ���������һ������
C����Һ����һ������ D���ɲ������»��ˮ�ķ���
��3������ͼ��֪�������е����Ե���������
��4������Ʒ�ڿ����лᷢ����ʴ����ԭ������������е�������ͬ���õĽ����Ϊ��ֹ������Ʒ����ʴ�������е������Ͳ˵�ͨ�������ķ�����������Ҫ��һ�ַ������ɣ���
��5����̽�������Ļ�ѧ����ʱ��ijͬѧ��������������ϡ�����У��۲쵽��������ʧ����Һ����ɫ��ɻ�ɫ���������ݲ������йط�Ӧ�Ļ�ѧ����ʽΪ������������
��6��С������FeSO4��CuSO4�������ʵ���Һ�У�����һ������þ�ۣ�����ַ�Ӧ����Һ��dz��ɫ�����ݴ����������������жϣ�����Ϊ��ȷ������������д��ţ���
A����ֽ��һ����Cu��������Fe��һ��û��Mg
B����ֽ��һ����Fe��������Mg��Cu
C����Һ��һ����FeSO4��������MgSO4��CuSO4
D����Һ��һ����MgSO4��FeSO4��һ��û��CuSO4��
��1����ͼ1��ˮ����ɲⶨ��ʵ�飬�˷�Ӧ�ķ���ʽΪ ��
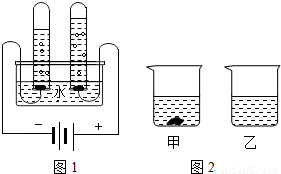
��2��ˮ�Ǻܺõ��ܼ����±���KNO3��KCl�ڲ�ͬ�¶�ʱ���ܽ�ȣ���ش��������⣺
| �¶�/�� | 10 | 20 | 30 | 40 | 50 | 60 | 70 | ||
| �ܽ��/g | KNO3 | 13.3 | 20.9 | 31.6 | 45.8 | 63.9 | 85.5 | 110 | 138 |
| KCl | 27.6 | 31.0 | 34.0 | 37.0 | 40.0 | 42.6 | 45.5 | 48.3 | |
�ڲ�������һ�ֲ����������������ձ��е�ʣ�����ȫ���ܽ⣬������Һ��Ϊ��������Һ������˵������ȷ���� ������ĸ����
A���ܼ��������ܲ��� B����Һ�����ʵ���������һ������
C����Һ����һ������ D���ɲ������»��ˮ�ķ���
��3������ͼ��֪�������е����Ե��� ��

��4������Ʒ�ڿ����лᷢ����ʴ����ԭ������������е� ��ͬ���õĽ����Ϊ��ֹ������Ʒ����ʴ�������е������Ͳ˵�ͨ�������ķ����� ��Ҫ��һ�ַ������ɣ���
��5����̽�������Ļ�ѧ����ʱ��ijͬѧ��������������ϡ�����У��۲쵽��������ʧ����Һ����ɫ��ɻ�ɫ���������ݲ������йط�Ӧ�Ļ�ѧ����ʽΪ �� ��
��6��С������FeSO4��CuSO4�������ʵ���Һ�У�����һ������þ�ۣ�����ַ�Ӧ����Һ��dz��ɫ�����ݴ����������������жϣ�����Ϊ��ȷ���� ����д��ţ���
A����ֽ��һ����Cu��������Fe��һ��û��Mg
B����ֽ��һ����Fe��������Mg��Cu
C����Һ��һ����FeSO4��������MgSO4��CuSO4
D����Һ��һ����MgSO4��FeSO4��һ��û��CuSO4��

