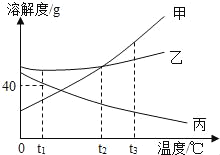
��Ŀ����
����Ŀ��ij����С���ͬѧ��������ѧҩƷ��ʱ������һƿ��ǩ�������ɫҺ�壬��ͼ��ʾ����ʦ���ߴ�ң���ƿҺ��������5��Һ���е�ijһ�֣�ϡH2SO4��H2O��MgCl2��Һ��NaOH��Һ��CuSO4��Һ��
��1������������Ϣ�жϣ�����ɫҺ��һ������_____��______��
��2��Ϊȷ����Һ��ijɷ֣�ͬѧ�����������ʵ�鷽��������һ�����ͬѧ�ǵ�̽�����
ʵ����� | ʵ������ | ʵ����� |
��ȡ��Һ���������Թ��У������еμ���ɫ��̪��Һ | ��Һ��ɫ�ޱ仯 | ��Һ�岻��______ |
����ȡ��Һ���������Թ��У������м���п�� | ______ | ��Һ����ϡH2SO4�� ����MgCl2��Һ |
����˼������
��3��ʵ��������з�����Ӧ�Ļ�ѧ������________��
���𰸡�H2O CuSO4��Һ NaOH��Һ �����ݲ��� ![]()
��������
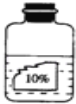
��������ʱ���ȿ������ʵ��������ʣ�������ͭ��Һ����ɫ�ģ�����������������ʹ��ɫ��̪��죬�����ܺ�п��Ӧ�������������
��1������������Ϣ����ǩ��ʾ���ʵ�����������10%�����Բ���ˮ����Һ����ɫһ����������ͭ��Һ����Ϊ����ͭ��Һ����ɫ�ģ�
��2����ȡ��Һ���������Թ��У������еμ���ɫ��̪��Һ����Һ��ɫ�ޱ仯����Һ�岻������������Һ����Ϊ����������ʹ��ɫ��̪��죻
����ȡ��Һ���������Թ��У������м���п���������������ݣ����Һ����ϡH2SO4������MgCl2��Һ����Ϊ�����ܺ�п�������ݣ�
��3��ʵ��������з�����Ӧ�Ļ�ѧ������![]() ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ���Ҵ��׳ƾƾ���������ҽ��������Ҳ����ȼ�ϡ�����ȫȼ�յĻ�ѧ����ʽ�ɱ�ʾΪ��C2H6O+3O2![]() 2 CO2+ 3 H2O ��
2 CO2+ 3 H2O ��
23g�Ҵ���ȫȼ�������Ķ��ٿ�������
�Ҵ�����ȫȼ�ջ����һ����̼��ijʵ���÷�Ӧǰ������ʵ��������±���
���� | �Ҵ� | ���� | ������̼ | ˮ | һ����̼ |
��Ӧǰ������g�� | 4.6 | 8.8 | 0 | 0 | 0 |
��Ӧ��������g�� | 0 | 0 | 6.6 | 5.4 | a |
������a��ֵΪ____ __��
����ʵ�鷢����Ӧ�Ļ�ѧ����ʽΪ��4C2H6O+11O2![]() _____ CO2+ _____ H2O + _____ CO ��
_____ CO2+ _____ H2O + _____ CO ��