��Ŀ����
����Ŀ��������ѧ��λͬѧΪ����ij�����Ͷ�����̼�Ļ���������Ƿ���CO�����ⶨ25gij����ͭ��Ʒ�Ĵ��ȣ��ֽ��û������ͨ������װ�ý��м���(��֪����ͭ��Ʒ�����ʲ����뷴Ӧ)��ʵ��װ����ͼ��ʾ��
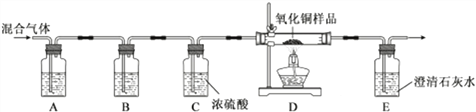
������ͼ�Իش�
(1)װ��A��Ӧʢ�ŵ��Լ���_______�������Ļ�ѧ��Ӧ�ķ���ʽ____________________��װ��B��Ӧʢ�ŵ��Լ���_________________��
(2)С������Dװ���к�ɫ��ĩ���ɫ��������Ϊ���������һ������CO��С����Ϊ�����뷨���ԣ���Ϊ����Ҳ���л�ԭ�ԡ�����Ϊ��������������У���Ҫ�۲쵽_______________________________����ʱ������֤�����������һ������CO��
(3)������ͭȫ������ԭ��С����Dװ������Ʒ������������4g�������Ʒ������ͭ����������Ϊ__________��
(4)С��ӻ��������ĽǶȿ��ǣ���Ϊ��ʵ������Ƿ�ף�����Ӧ����θĽ�?__________________��
���𰸡� ����������Һ 2NaOH + CO2 == Na2CO3 + H2O ����ʯ��ˮ E�г���ʯ��ˮ����� 80% ��E�����һ���ƾ��ƻ���E�Ҷ˵�������һ������
����������1��Ҫ����һ����̼�Ĵ��ڣ���������һ����̼������ͭ��Ӧ���ɶ�����̼������Ҫ��������ԭ�еĶ�����̼ͨ���������Ƴ�ȥ��Ȼ��B��ʢ�г����ʯ��ˮ֤��������̼ȫ������ȥ�����������������̼��Ӧ����̼���ƺ�ˮ����ѧ����ʽΪ��2NaOH+CO2�TNa2CO3+H2O��
��2������������������ͭ��Ӧ������Ҫͨ���۲�E�г���ʯ��ˮ�����֤���ж�����̼���ɣ��Ӷ�˵������һ����̼��
��3��Dװ������Ʒ������������4g��˵�����ٵ���Ԫ�ص�����Ϊ4g��������ͭ������Ϊ�� 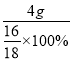 =20g��������ͭ����������Ϊ��
=20g��������ͭ����������Ϊ�� ![]() ��100%��80%��
��100%��80%��
��4��һ����̼���ж������壬��Ҫ����β��������������E�����һ���ƾ��ƻ���E�Ҷ˵�������һ������

 ���ĺ����Ͼ�������ϵ�д�
���ĺ����Ͼ�������ϵ�д�