��Ŀ����
����Ŀ��ij��ѧ��ȤС��������ͼ��ʵ��װ��̽����ȼ��ȼ�����������ԭ����

���������ϣ�
���������װ����ж�����̼��˵ĺ������������Ż��40�棬�����Ż��240�档
��ʵ������ʵ��
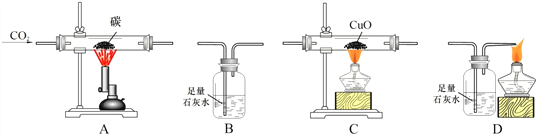
��.����ͬѧ����ͼ��װ�ý���ʵ�飬�۲쵽���µ�ʵ������
A.��ͭƬ�Ϻ��ײ�ȼ�� B.��ˮ�еİ��ײ�ȼ�� C.��ͭƬ�ϰ���ȼ��
��������ʵ�������������:
�ٸ���ʵ������_______________�Աȵó�:��ȼ��ȼ����Ҫ�������Ӵ���
�ڸ���ʵ������______________�Աȵó�����ȼ��ȼ���¶�Ҫ�ﵽ�����Ż�㡣
��.����ͬѧ����ͼ�ҽ���ʵ�飨�г�������ȥ����ʵ����̣�
��ͨ��N2����ȼ�ƾ��ƣ�һ��ʱ���a��b�о�����������
��ֻ����b����ܣ�������ͨ02��a������������b�к�zȼ�ա�
��1��ʵ������У���˵����ȼ��ȼ����Ҫ������������____________________________��
��2��ʵ����̢��У��Ա�a��b�е�ʵ������֪��ȼ��ȼ�յ�����֮һ��_______________________��
����˼����չ��
��1������ʵ�����ͨ��N2��Ŀ����__________________________________________��
��2������ȼ�յĻ�ѧ����ʽΪ____________________________��
��3���ӻ����Ƕȷ�����______________����ס����ҡ�����ʵ������㡣
��4��ͼ��Ϩ���������ķ����У����á����¶Ƚ����Ż�����¡�ԭ������______________������ţ���
���𰸡�B��C A��C �������bͨN2����ȼ�գ��������bͨO2��ȼ�� ȼ����Ҫ�¶ȴﵽ��ȼ����Ż�� �Ա�˵��ȼ����Ҫ���� 4P+5O2![]() 2P2O5 �� ��
2P2O5 �� ��
��������
[ʵ������ʵ]1������ͬѧ����ͼ��װ�ý���ʵ�飬�۲�Ա�ʵ�����ٸ���ʵ��������ˮ�еİ��ײ�ȼ�պͱ�ͭƬ�ϰ���ȼ�նԱȵó�����ȼ��ȼ�գ���Ҫ�������Ӵ���
�ڸ���ʵ������ͭƬ�Ϻ��ײ�ȼ�պͱ�ͭƬ�ϰ���ȼ�գ��Աȵó�����ȼ��ȼ�գ��¶�Ҫ�ﵽ�����Ż�㣻
2����1��ʵ������У���˵����ȼ��ȼ����Ҫ�����������Dz������bͨN2����ȼ�գ��������bͨO2��ȼ�գ�
��2��ʵ����̢��У��Ա�a��b�е�ʵ������a�еĺ��ײ�ȼ�գ�b�еĺ�����ȼ�տ�֪����ȼ��ȼ�յ�����֮һ��ȼ����Ҫ�¶ȴﵽ��ȼ����Ż�㣻
[��˼����չ]��1������ʵ�����ͨ��N2����ȼ�ƾ��ƣ�һ��ʱ���a��b�о�����������ͨ��������ֻ����b����ܣ�b�к���ȼ�գ�˵����ͨ��N2��Ŀ���ǣ��Ա�˵��ȼ����Ҫ������
��2������ȼ���������������ף���Ӧ�Ļ�ѧ����ʽΪ��4P+5O2![]() 2P2O5��
2P2O5��
��3���ӻ����Ƕȷ�����ͼ����ʵ������㣬�����Dz���Ⱦ������
��4��ͼ��Ϩ���������ķ����У����������¶Ƚ����Ż����������ԭ�����Ǣ����촵��

����Ŀ��п�̵�أ��׳Ƹɵ�أ��������е������ܴ��乹����ͼ1��ʾ��
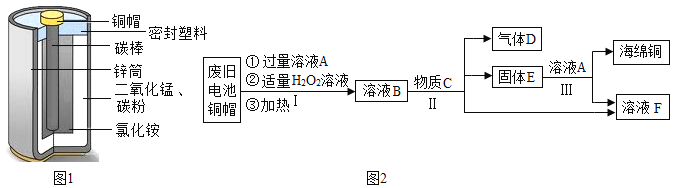
��1������ͼ1�жϣ����ڽ������ϵ���______��
��2��������Ʒ�ڿ����з��������⣬���������������______ͬʱ�Ӵ����Ͼɵ��ͭñ������һЩ��ɫ��ͭ�⣬����Ҫ�ɷ�Ϊ��Cu2��OH��2CO3���Աȿ�֪ͭ�������������������������п����е�______���롣
��3�����÷Ͼɵ��ͭñ����Cu��Zn����ȡ����ͭ��Cu�������õ�����п��Һ����Ҫ������ͼ2��ʾ
��ͬѧ�Dz�������֪��Cu+H2O2+H2SO4=CuSO4+2X�����ƶ�x�Ļ�ѧʽ______��
��д�����������漰��Ӧ�Ļ�ѧ����ʽ��______����д��һ�����ɣ�
������˵����ȷ����______��
A������I��H2O2Ҳ�п��ֽܷ���������
B������E�ǻ����
C����ҺF����ҺB�к�����п������������
��4��ͬѧ��ͨ����������֪����пƤΪ����п����������������
��ȡһ��пƤ����ʢ��һ����CuSO4��Һ���ձ��У���ַ�Ӧ��õ���ҺX����Y��������Y��ֻ��һ������ʱ����ҺX��һ�����е�����Ϊ______����д����ѧʽ��
����ȡһ������Ϊ6.5gпƤ������ϡ������ȫ��Ӧ������������������______0.2g��ѡ��������������������=������
��5����ȡ��3���е�ͭñ20�������ձ��У����ձ����������μ���ϡ���ᷴӦ������¼���������������ʵ���������£�
��һ�� | �ڶ��� | ������ | |
��������ϡ�����������g�� | 40 | 40 | 40 |
�����������������g�� | 0.08 | 0.16 | 0.20 |
��������ϡ���������ʵ���������Ϊ______��д��������̣�����������һλС������
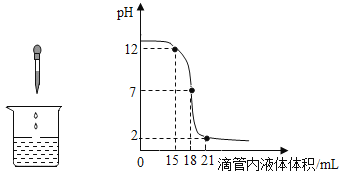
����Ŀ����ͼ��ܷ����кͷ�Ӧ�������ճ������ũҵ���������Ź㷺��Ӧ�ã���ͼ��ʾϡ���������������Һ������Ӧʱ�ձ�����Һ��pH�����Һ������ı仯������ص�ʵ�����������л�ȡ��Ϣ���ش��������⡣
(1)���ձ���ʢ�ŵ���_____��Һ��
������������Ϊ(15��12)�ĵ�����ʾ�����壺_____��
(2)С���������ʵ�鼸����֣����Ǹ���ʢ������������Һ�Լ�ƿ��ƿ���������С���������ʵ�鷽������������������Һ�Ƿ���ʡ�
(��ʾ��̼�������Ȼ�����Ӧ������̼�ᱵ�������Ȼ���)
ʵ�鷽�� | ʵ�鲽�� | ʵ������ | ʵ����� |
����һ | ȡ������Һ���Թ��У��μӼ���ϡ���� | _____ | û�б��� |
������ | ȡ������Һ���Թ��У��μӼ����Ȼ�����Һ | ������ɫ���� | �Ѿ����� |
����Ϊ����һ��ʵ������Ƿ���ȷ���������жϲ�˵�����ɣ�_____��