��Ŀ����
����Ŀ����1��������ˮ��ʳ��������������벻��������
��������Ϊ�ܵ��¿�����PM2.5�������ӵ���______________������ĸ����
a.����ʹ�û�ʯȼ�� b.¶��������� c.��չ�����ͨ����̼����
�ں���������ʳ�����ǵĽ������������ʹ��䣺ţ�̡���������͡�ˮ���ȣ����к��е�Ӫ��������֬�����ࡢ___________��ά���ء����κ�ˮ�ȡ�
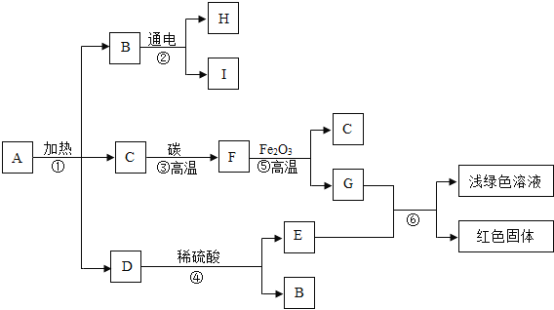
��2������Ƚ����еġ����Ȼ��������������������㡣�����Ȼ������Ҫ���ϰ��ͷ��Ȱ�����Ҫ�ɷ�Ϊ��ʯ�ҡ����չ�����������̿�����ۡ����ۡ�̼���Ƶȣ���ɣ�ʳ�÷�����ͼ��ʾ����ش�
�ٷ��Ȱ����ȵ�һ��ԭ������ʯ����ˮ��Ӧ���ȡ���Ӧ�Ļ�ѧ����ʽΪ____________��
�ڱ��չ�����������̿�������ɶ�Ľṹ������______________���á�
�ۡ����Ȼ�������ڡ�����ɾ۱�ϩ���ϲ����Ƴɣ����ⶪ�������______________���⡣
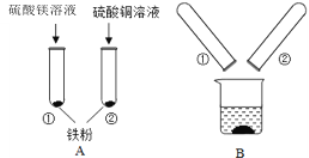
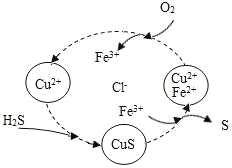
��3��������顶�����ӡ����أ��������ࣨ����ͭ��Ϳ��������ɫ��ͭ�������û�ѧ����ʽ��ʾ��ԭ��____________��
��4������ĩ������Ĺ��չ��顶�����ǡ������С��������ķ����������á��ң���ľ�ң��͡��ס������ǻң���ϼ�ˮ���õ���Һ��ϴ��˿����������Һ��ϴ��˿����Ҫ����Ϊ���к���______________��д��ѧʽ����
���𰸡�ab ������ CaO + H2O = Ca��OH��2 ���� ��ɫ��Ⱦ CuSO4+ Fe = Cu + FeSO4 KOH
��������
��1���ٴ���ʹ�û�ʯȼ�Ϻ�¶����������ܵ��¿�����PM2.5�������ӣ���չ�����ͨ����̼���п��Լ��ٿ�����PM2.5���������ӡ����ab��
��ţ�̸��������������Ρ�ˮ�ȡ�����������ࡢ��������֬��ˮ������ά���صȡ���������ʡ�
��2������ʯ����ˮ��Ӧ�����������ƣ�ͬʱ�ų���������Ӧ�Ļ�ѧ����ʽΪ��CaO + H2O = Ca��OH��2��
�����չ�����������̿�������ɶ�Ľṹ�������������á�
�ۡ����Ȼ�������ڡ�����ɾ۱�ϩ���ϲ����Ƴɣ����ⶪ���������ɫ��Ⱦ���⣻
��3�������ࣨ����ͭ��Ϳ��������ɫ��ͭ�ķ�Ӧ����������ͭ��Ӧ��������������ͭ����Ӧ�Ļ�ѧ����ʽΪ��CuSO4+ Fe = Cu + FeSO4��
��4����ľ�ҵ���Ҫ�ɷ�Ϊ̼��أ����ǻҵ���Ҫ�ɷ�Ϊ�����ƣ�.�����ƺ�ˮ��Ӧ�����������ƣ��������ƺ�̼��ط�Ӧ����̼��Ƴ������������أ����������Һ��ϴ��˿����Ҫ����Ϊ����һ����KOH��

 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�