��Ŀ����
���������ڡ�ˮ���ϣ���������70.8%��ˮ���ǡ�
��1����ˮɹ���ǽ����չ�ͷ���ʹ��ˮ�е�_________��д��ѧʽ���������õ����н϶����ʵ�____��д��ѧʽ������.
��2����������Ȼ����ˮѭ����һ�����ڣ�____________�������Щ�����ڿ����з�Ӧ���������������ˮ�����γ����ꡣA��B��C����������ˮ��pH��ͼ��ʾ������_________��������ˮ�����ꡣ
��3���밴Ҫ��д�����л�ѧ�̲���ˮ��Ϊ��Ӧ��Ļ�ѧ����ʽ���������Ϸ�Ӧ��������������벻ͬ����
�ٷֽⷴӦ��_______________________________________________��
�ڻ��Ϸ�Ӧ��_______________________________________________��
�ۻ��Ϸ�Ӧ��_______________________________________________��
��1��H2O��NaCl ��2����������AB ��3����2H2O 2H2��+O2��
2H2��+O2��
��CO2+H2O��H2CO3 ��CaO+H2O��Ca(OH)2
���������������1����ˮɹ���ǽ����չ�ͷ���ʹ��ˮ�е�ˮ�������õ����н϶����ʵ��Ȼ��ƾ��壬ˮ���Ȼ��ƵĻ�ѧʽ�ֱ���H2O��NaCl��
��2�������������������ʹ�ú������ܸߵ�ú��ʯ�͵Ȼ�ʯȼ�ϣ�ȼ�պ�����Ķ��������������ȣ��ڴ����о������ӵĻ�ѧ��Ӧ��������ˮ���γ����ꣻ�����������������γ��������Ҫ���ʡ�pH��5.6����ˮ�������꣬��A��B��C����������ˮ��pHͼ��A��B������ˮ��PH��5.6���������ꡣ
��3����ˮͨ��ֽ��������������������ڷֽⷴӦ����Ӧ�Ļ�ѧ����ʽΪ2H2O 2H2��+O2����
2H2��+O2����
�ڶ�����̼��ˮ��Ӧ����̼�ᣬ���ڻ��Ϸ�Ӧ����Ӧ�Ļ�ѧ����ʽΪCO2+H2O��H2CO3��
�������ƺ�ˮ��Ӧ�����������ƣ����ڻ��Ϸ�Ӧ����Ӧ�Ļ�ѧ����ʽΪCaO+H2O��Ca(OH)2��
���㣺���麣ˮɹ�ε�ԭ�����̣�����IJ�����Σ�������Σ���д��ѧ����ʽ�����ֱ���ʽ

������������������Ҫ�����ʡ�
��1�������кܶ���;����������������;����____________������ţ��� 
| A��ҽ�Ƽ��� | B��ʳƷ���� | C�������� | D����� |
��3������ͼ��ʾװ�òⶨ�����е�����������
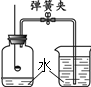
�����Ѿ������װ�õ������ԣ�����ǰ��Ӧ������������_______________________________��
�ڵ�ȼ���ײ����뵽����ƿ�У��ɹ۲쵽______________________________________����Ӧ�����ֱ���ʽΪ___________________________________________________��
�۲�����ľ̿����۴������ʵ���ԭ����____________________________________��
 2CO2 2Mg + O2
2CO2 2Mg + O2


