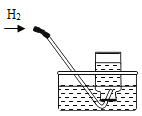
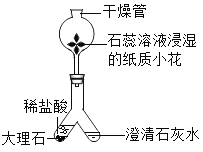
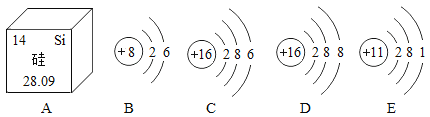
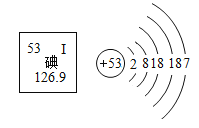
��Ŀ����
����Ŀ���ҹ�Լ���ϱ���ʱ�Ϳ�ʼұ����ͭ����ͭ��ͭ��п�ĺϽ�Cu��Zn������������������������������Ʒ��С������һƿϡ�������ص��������ⶨ��ͭ��Ʒ����ɣ�������ͭ�е��������ʣ�����30mlϡ����ֱ���뵽10g��ͭ��ĩ�У�ÿ�γ�ַ�Ӧ�ⶨ����������������ʵ�����ݼ��±���
��һ�� | �ڶ��� | ������ | |
����������������/mL | 10 | 10 | 10 |
��������������/g | 0.08 | 0.08 | 0.04 |
(1)���ϱ����ݷ�����С����10g�Ͻ��ĩ���ռ������� g��
(2)��úϽ���ͭ������������
���𰸡���1��0.2 ��2��35%
����������1�������β�����������������Ϊ10g�Ͻ��ĩ�ܹ��ռ�����������������0.08g+0.08g+0.04g�T0.2g��
��2���⣺��úϽ���п������Ϊx��
Zn+2HCl�TZnCl2+H2��
65 2
x 0.2g
![]()
���x=6.5g
�Ͻ���ͭ������Ϊ10g-6.5g�T3.5g
�úϽ���ͭ����������Ϊ��![]() ��100%=35%
��100%=35%
�𣺸úϽ���ͭ����������Ϊ35%��

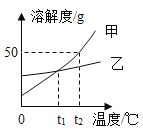
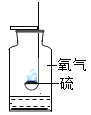
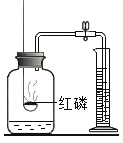
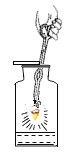
����Ŀ������ʵ��ָ�������е�ˮ�������û������ˮ����Ҫ���õ��ǣ� ��
A | B | C | D | |
ʵ �� װ �� |
����������ȼ�� |
�ⶨ�������������� |
��˿��������ȼ�� |
��ˮ���ռ����� ������������ˮ�� |
���� | ����ƿ�е�ˮ:���շų������� | ��Ͳ�е�ˮ:ͨ��ˮ����ı仯�ó�O2��� | ����ƿ�е�ˮ����ȴ�����������ֹ����ƿը�� | ����ƿ�е�ˮ��ˮ�Ƚ�����ƿ�ڵĿ����ž�������ڹ۲�������ʱ�ռ��� |
A.AB.BC.CD.D