��Ŀ����
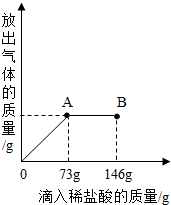 ��֪ Na2CO3��ˮ��Һ�ʼ��ԣ���һ�ձ���ʢ��20.4g Na2CO3��NaCl ��ɵĹ�������������μ������ʷ���Ϊ10%��ϡ���ᣮ�ų��������������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺
��֪ Na2CO3��ˮ��Һ�ʼ��ԣ���һ�ձ���ʢ��20.4g Na2CO3��NaCl ��ɵĹ�������������μ������ʷ���Ϊ10%��ϡ���ᣮ�ų��������������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺��1�����μ�ϡ������ͼ��B��ʱ���ձ�����Һ��pH
��2����ʵ����ɺų����������Ϊ���٣�
��3�����μ�ϡ������ͼ��A��ʱ���ձ���Ϊ��������Һ�����£���ͨ����������������ʵ��������������������� ��һλС����
ע�������õ������ԭ��������H��1 Cl��35.5 O��16 Na��23 C��12��
����������Ҫ����ͼ����ͼ��֪�����μ�ϡ���ᵽA��ʱ��̼������ȫ��Ӧ����Ӧ���������Ȼ��ƣ�ԭ���Ĺ���������Ҳ���Ȼ��ƣ����������Ȼ����������������������е�̼���Ƶ��������Ӷ�����������������Ȼ��Ƶ��������ټ��Ϸ�Ӧ�����ɵ��Ȼ��Ƶ��������Ƿ�Ӧ�����ʵ�����������Ӧ����Һ������Ϊ�����������ʵ�������ȥ���ɵ������������
����⣺��1����ͼ��֪�����μ�ϡ������A��ʱ��������ȫ��Ӧ�����Ե��μӵ�B��ʱ������������ձ�����Һ��PH��7���ʴ�Ϊ������
��2���⣺��μ�ϡ���ᵽAʱ���ų����������ΪX���μӷ�Ӧ��̼���Ƶ�����ΪY�����ɵ��Ȼ��Ƶ�����ΪZ
�������֪�����μӵ�Aʱ�������Ȼ����������73g��10%�T7.3g
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 73 117 44
Y 7.3g Z X
�T
��Y�T10.6g
�T
��Z�T11.7g
�T
��X�T4.4g
����Ϊ�Ȼ��ƣ��Ȼ��Ƶ�����Ϊ��11.7g+20.4g-10.6g�T21.5g
��Һ������Ϊ��20.4g+73g-4.4g�T89g
�������ʵ���������Ϊ��
��100%�T24.2%
�𣺣�2����ʵ����ɺ��������������Ϊ4.4g ��3�����μ�ϡ������ͼ��A��ʱ�����ʵ���������Ϊ24.2%
��2���⣺��μ�ϡ���ᵽAʱ���ų����������ΪX���μӷ�Ӧ��̼���Ƶ�����ΪY�����ɵ��Ȼ��Ƶ�����ΪZ
�������֪�����μӵ�Aʱ�������Ȼ����������73g��10%�T7.3g
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 73 117 44
Y 7.3g Z X
| 106 |
| Y |
| 73 |
| 7.3g |
| 117 |
| Z |
| 73 |
| 7.3g |
| 44 |
| X |
| 73 |
| 7.3g |
����Ϊ�Ȼ��ƣ��Ȼ��Ƶ�����Ϊ��11.7g+20.4g-10.6g�T21.5g
��Һ������Ϊ��20.4g+73g-4.4g�T89g
�������ʵ���������Ϊ��
| 21.5g |
| 89g |
�𣺣�2����ʵ����ɺ��������������Ϊ4.4g ��3�����μ�ϡ������ͼ��A��ʱ�����ʵ���������Ϊ24.2%
���������ݷ�Ӧ�Ļ�ѧ����ʽ�ܱ�ʾ��Ӧ�и����ʵ�������ϵ���ɷ�Ӧ��ij���ʵ������ɼ������Ӧ���������ʵ�������

��ϰ��ϵ�д�
 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
�����Ŀ
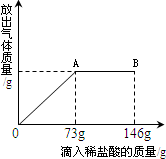 ��֪Na2CO3��ˮ��Һ�ʼ��ԣ���һ�ձ���ʢ��31.4g Na2CO3��NaCl��ɵĹ�������������μ�������������Ϊ10%��ϡ���ᣬ�ų��������������������ϡ�����������ϵ��������ͼ��ʾ�����������ش����⣺
��֪Na2CO3��ˮ��Һ�ʼ��ԣ���һ�ձ���ʢ��31.4g Na2CO3��NaCl��ɵĹ�������������μ�������������Ϊ10%��ϡ���ᣬ�ų��������������������ϡ�����������ϵ��������ͼ��ʾ�����������ش����⣺ ��2013?�����ض�ģ����֪Na2CO3��ˮ��Һ�ʼ��ԣ���һ�ձ���ʢ��20.4g Na2CO3��NaCl��ɵĹ�������������μ������ʷ���Ϊ10%��ϡ���ᣮ�ų��������������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺
��2013?�����ض�ģ����֪Na2CO3��ˮ��Һ�ʼ��ԣ���һ�ձ���ʢ��20.4g Na2CO3��NaCl��ɵĹ�������������μ������ʷ���Ϊ10%��ϡ���ᣮ�ų��������������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺ ��֪Na2CO3��ˮ��Һ�ʼ��ԣ���һ�ձ���ʢ��20g Na2CO3��NaCl��ɵĹ�������������μ�������������Ϊ10%��ϡ���ᣬ�ų��������������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺
��֪Na2CO3��ˮ��Һ�ʼ��ԣ���һ�ձ���ʢ��20g Na2CO3��NaCl��ɵĹ�������������μ�������������Ϊ10%��ϡ���ᣬ�ų��������������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺