��Ŀ����
����Ŀ��ʯ��ʯ�dz��õĽ������ϡ�
�ټ���ͬѧ���������ʵ�鷽������̽����
��.��ͬѧȡʯ��ʯ����ͼ��ʾ����ʵ�飨ú�����ܴﵽʯ��ʯ�ֽ���¶ȣ����۲쵽�ձ��ڱ�ʯ��ˮ����ǣ��ɴ˼�ͬѧ��Ϊʯ��ʯ�ѷֽ⡣��ͬѧ���۲�������������__________��
��.��ͬѧ��������ʵ�飬����±�
ʵ�鲽�� | ʵ������ | ʵ����� |
ȡ�������պ�������Թ��У���ˮ�����ˣ�ȡ��Һ���μ�____________��Һ | ��Һ��� | ֤�������� ����________ |
ȡ�����μ�������ϡ���� | _______ | ����̼��� |
��Ϊ�˲ⶨʯ��ʯ��̼��Ƶ������������������ʲ��μӷ�Ӧ������ͬѧ��Ƴ�ȡһ��������ʯ��ʯ��Ʒ�������������������ٸı䡣ʵ���й���������仯���£�
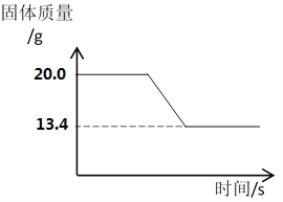
��.���ɶ�����̼��������____________g��[
��.��ʯ��ʯ��̼��Ƶ����ʵ����������ݻ�ѧ����ʽ��д��������̣�___________
���𰸡� һ����̼ȼ��Ҳ�ܲ���������̼��ʹʯ��ˮ����ǵĶ�����̼��һ��������ʯ��ʯ�ֽ� ��̪ ������ ������ 6.6 0.15 mol
������������һ��ʵ��̽���⣬�٢�.�۲쵽�ձ��ڱ�ʯ��ˮ����ǣ���Ϊʯ��ʯ�ѷֽ⡣���۲�������������һ����̼ȼ��Ҳ�ܲ���������̼��ʹʯ��ˮ����ǵĶ�����̼��һ��������ʯ��ʯ�ֽ⣬ú�������ȼ�տ��Բ���һ����̼����.
ʵ�鲽�� | ʵ������ | ʵ����� |
ȡ�������պ�������Թ��У���ˮ�����ˣ�ȡ��Һ���μӷ�̪��Һ����̪���Լ�����Һ���ɫ�� | ��Һ��� | ֤�������� ���������ƣ���������ˮ��Ӧ�����������ƣ���ˮ��Һ�ʼ��ԡ� |
ȡ�����μ�������ϡ���ᣬ��̼��Ʒ�Ӧ�������塣 | ������ | ����̼��� |
�ڲⶨʯ��ʯ��̼��Ƶ�������������ͼ����Ϣ֪����.���������غ㶨�ɣ����ɶ�����̼�������ǡ�20g��13.4g��6.6g����.�⣺��ʯ��ʯ��̼��Ƶ����ʵ���Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
X 6.6g
![]() ��
��![]() ��x��15g
��x��15g
![]() ��0.15 mol��
��0.15 mol��
�㾦��ú�������ȼ������һ����̼��һ����̼���п�ȼ�ԣ�ȼ�����ɶ�����̼�����ɶ�����̼��ʹ����ʯ��ˮ����ǡ�

����Ŀ��ͬѧ�ǿ���ͨ�����з�ʽ��ʶ������
����ɽǶȡ�
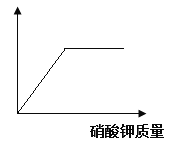
�ٿ������������ԼΪ78%��������___________��
��Ϊ�ⶨ������������������������ͼʵ�顣
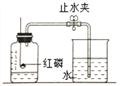
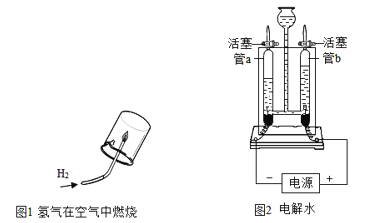
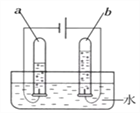
��.Ϊ��ȷ��ʵ��ɹ�����װҩƷ֮ǰӦ�ü��װ�õ� _________��
��.��ʵ���к�����Ҫ������ԭ����__________��
��.����ȼ�յ�������____________����Ӧ�Ļ�ѧ����ʽ ____________��
��.��ȴ�����º��ֹˮ�й۲쵽�������� _______________���ɴ˵ó��������������������ԼΪ ___________��
���۽Ƕ� ��
���á���ѧ���š���ͼʾ����ա�
![]()
ͼ ʾ |
| ______ |
|
��ѧ���� | ______ | N2 | _______ |
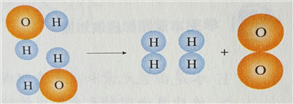
��ͬ��ͬѹ�£����������ȵ��ڷ��Ӹ����ȡ������Կ����������ɷ֣���ͼ�ɱ�ʾ������ģ�͵���__________(��ѡ��)��
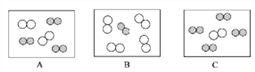
���仯�Ƕȡ�
��һ��������ѹ�£������в�����ֵķе����£�
��� | ���� | ���� | ������̼ |
�е�(��) | -195.8 | -183.0 | -78.4 |
�ٽ�ȼ�ŵ�ľ������ʢ�б�����ֵĻ��Һ�ĸ�ƿ�ڣ��۲쵽��������____________��
�����������������_____________��
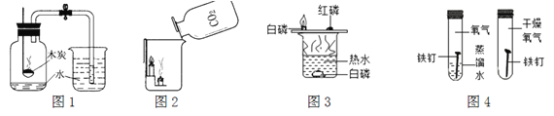
A��ľ̿��������ȼ�գ�������
B����˿�ڿ�����ȼ�գ��������䣬���ɺ�ɫ����
C���ӱ������ó���ˮ��ƿ�������Һ�飬˵����������ˮ����
D�����ó���ʯ��ˮ���Լ�ƿ�ڱ���һ���Ĥ��֤���������ж�����̼
����˿��������ȼ�յĻ�ѧ����ʽ��____________��
��Ӧ�ýǶȡ�
�پƾ�(C2H5OH)��һ�ֳ������������ƾ���___________��Ԫ����ɣ�������Ԫ������Ԫ�ص�������Ϊ_______����Ԫ�ص���������Ϊ________(���÷�����ʾ)��ÿ���ƾ����Ӻ�________��ԭ�ӣ�46gC2H5OH�к�_________����ԭ�ӡ���ƽ�ƾ�ȼ�յĻ�ѧ����ʽ��ϵ������Ϊ_______��
��C2H5OH+��O2![]() ��CO2+��H2O
��CO2+��H2O
��ʳƷ��װ�ڳ�N2�Է�������ΪN2�Ļ�ѧ����_____________��
����Ŀ��������ʦ������ƿ�ޱ�ǩ���Լ���һƿ�ǹ���һƿ��Һ�塣��ʦ������ȡ����������Թ���������һ����ɫ���塣���Ƕ���������˶���̽����
(1)�ٸ��������������ʣ���֤���ķ������£�
�� �� | �� �� �� �� | �� �� �� �� �� |
�����������__________ | ________________ | ______________ |
����������Ļ�ѧ����ʽ������____________________________________
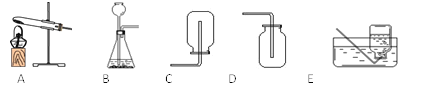
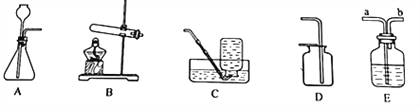
��������ȡ�����壬���õķ���װ�ÿ�ѡ����ͼ�е�_____���ռ�װ����____��(2)�Ҳ������ɫ���廹��������һ�����壬��ȡ������Ļ�ѧ����ʽ��_____________________________��
(3)ʵ���ҿ�����ʯ�Һ��Ȼ�����ֹ�����ȷ�Ӧ��ȡ������������һ�ּ�������ˮ���ܶȱȿ���С�����塣��������������ʵ������ȡ����Ӧ��ѡ�õķ���װ����_________���ռ�װ��ѡ��__________��