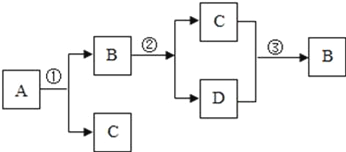
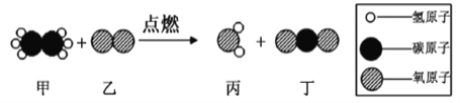
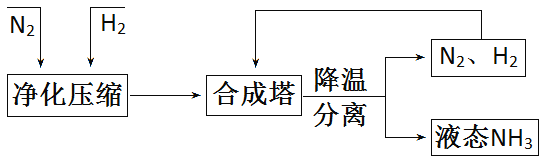
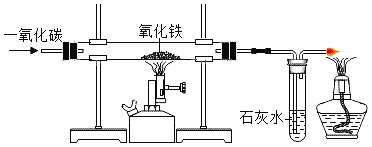
��Ŀ����
����Ŀ����ѧ������ѧϰ��ѧ����Ҫ���ߡ�
��1���û�ѧ�����ʾ
����������_____ �� ��̼�������____________��
��ͭԭ��_________ �� ������������Ԫ�صĻ��ϼ�___________��
��2������������ĸ�����գ�a��CO2 b��NaCl c��N2 d��Fe2O3
���������Ҫ�ɷ���___________ �� �ڸɱ�����Ҫ�ɷ���____________��
�۳�����������ζ������___________ ���ܳ�����ʳƷ����������____________��
��3������ǧ������д�����з�Ӧ�Ļ�ѧ����ʽ��
����ʯ�ұ����ʯ��___________ �� ����˿��������ȼ��___________��
������ͭ������������Һ��Ӧ_________ �� ����������������θ�����_______��
���𰸡� H2 CO32- Cu ![]() d a b c CaO + H2O = Ca(OH)2 �������10��4Fe + 3O2
d a b c CaO + H2O = Ca(OH)2 �������10��4Fe + 3O2 ![]() Fe3O4 CuSO4 +2 NaOH = Cu(OH)2 �� + Na2SO4 Al(OH)3 + 3HCl = AlCl3 + 3H2O
Fe3O4 CuSO4 +2 NaOH = Cu(OH)2 �� + Na2SO4 Al(OH)3 + 3HCl = AlCl3 + 3H2O
����������1���ٻ�ѧʽ����Ԫ�ط��ź����½ǵ������������ʾ����Ҫ��ʾ������ӣ������ڻ�ѧʽ��ǰ��������ֱ�ʾ���ӵĸ�����������1ʱ����ʡ�Բ�д���������ӱ�ʾΪH2��
����������ԭ�ӻ�ԭ���ź����Ͻǵ����ּ�����������ʾ��������ǰ���������ں�������1ʱ����ʡ�Բ�д��̼������Ӵ�2����λ�ĸ���ɣ���ʾΪCO32-��
��ԭ��ֱ����Ԫ�ط�������ʾ��ͭԭ�ӱ�ʾΪCu��
���������Ļ�ѧʽΪAl2O3����Ԫ�صĻ��ϼ�Ϊ-2�ۣ����ݻ��ϼ۵Ĵ�����Ϊ0,����Ԫ�صĻ��ϼ�Ϊ+3�ۣ���ʾΪ�� ![]() 2
2![]() ;
;
��2�����������Ҫ�ɷ�������������ѧʽΪFe2O3������d��
�ڸɱ��ǹ�̬�Ķ�����̼����ѧʽΪCO2������a��
�۳�����������ζ�����Ȼ��ƣ���ѧʽΪNaCl������b��
�ܳ�����ʳƷ�������ǵ�������ѧʽΪN2������c��
��3������ʯ����ˮ��Ӧ�����������ƣ���Ӧ����ʽΪ��CaO+H2O==Ca(OH)2��
����˿��������ȼ��������������������Ӧ����ʽΪ��3Fe+2O2��ȼFe3O4��
������ͭ���������Ʒ�Ӧ����������ͭ�����������ƣ���Ӧ����ʽΪ��2NaOH+CuSO4=
Cu��OH��2![]() +Na2SO4;
+Na2SO4;
����������������θ�������������������ϡ���ᷴӦ�����Ȼ�����ˮ����Ӧ����ʽΪ��
Al��OH��3+3HCl=AlCl3+ 3H2O��

����Ŀ���Ȼ��ƺ�����þ���ܽ�������������¶��µ��ܽ�ȱ����£�
�¶�/�� | 20 | 30 | 40 | 60 | 80 | 90 | 100 | |
�ܽ�� (g/100GH20) | NaCl | 36.0 | 36.3 | 36.6 | 37.3 | 38.4 | 39.0 | 39.8 |
MgSO4 | 33.7 | 38.9 | 44.5 | 54.6 | 55.8 | 52.9 | 50.4 | |
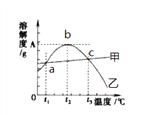
��ش��������⣺
(1)80��ʱ������þ���ܽ��Ϊ__________���ܽ��������������������__________��a���Ӧ���¶�t1��Χ��____________________��
(2)t2��ʱ�к��н϶�NaCl ��MgSO4��Һ��Ϊ�˵õ�������MgSO4���ɲ��õķ���__________
(3)40��ʱ��20g NaCl����50gˮ�У��γɵ���Һ��������������Ϊ__________(��ȷ��0.1%)��Ҫʹt3�汥�͵�MgSO4��Һ�����������������ɲ��õĴ�ʩ��__________��