��Ŀ����
�ڿ����У�ͬѧ�������ᡢ���ᱵ���������ơ�̼����������ʵ���Һ�����и��ֽⷴӦ����������̽����
��1��ͨ���ж�����������Һ����֮���ܷ�����Ӧ��д�����з������������Ļ�ѧ����ʽ��
�г������ɵķ�Ӧ��
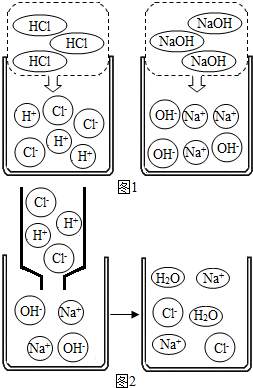
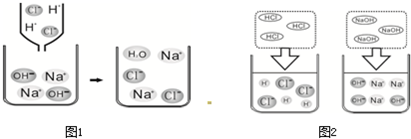
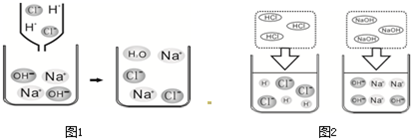
��2������ͼ1��HCl��NaOH��ˮ�н������ʾ��ͼ��ͼ2��ϡ������������Ʒ�Ӧ���۹���ʾ��ͼ��
��������������Ʒ�����Ӧ����ʵ����ʲô��
��ͨ�������ϵ�̽���ܷ��֣�����Һ�У�����H+��OH-���ܴ��������⣬
��3�����������������ʵ���Һ���ʵ����������ʻ�ϲ����ˣ��õ�һ����ɫ��Һ��
�پ��ⶨ������Һ�����ԣ������Һ��һ�����е�������
��Ϊ��֤����Һ�п��ܺ��е������Ƿ���ڣ����������ʵ�������֤������֤һ�����Ӽ��ɣ�
��4��100gϡ����ǡ�ø�100g������������Ϊ16%������������Һ��ȫ��Ӧ����������Һ�����ʵ�����������
��1��ͨ���ж�����������Һ����֮���ܷ�����Ӧ��д�����з������������Ļ�ѧ����ʽ��
�г������ɵķ�Ӧ��
H2SO4+Ba��OH��2=BaSO4��+2H2O
H2SO4+Ba��OH��2=BaSO4��+2H2O
����2������ͼ1��HCl��NaOH��ˮ�н������ʾ��ͼ��ͼ2��ϡ������������Ʒ�Ӧ���۹���ʾ��ͼ��

��������������Ʒ�����Ӧ����ʵ����ʲô��
�����Ӻ���������������ˮ���ӻ�H++OH-=H2O
�����Ӻ���������������ˮ���ӻ�H++OH-=H2O
����ͨ�������ϵ�̽���ܷ��֣�����Һ�У�����H+��OH-���ܴ��������⣬
Ba2+��SO42-
Ba2+��SO42-
�����������ӷ��ţ�Ҳ���ܴ������森��3�����������������ʵ���Һ���ʵ����������ʻ�ϲ����ˣ��õ�һ����ɫ��Һ��
�پ��ⶨ������Һ�����ԣ������Һ��һ�����е�������
K+��NO3-��H+��Na+
K+��NO3-��H+��Na+
��������д4�����ӷ��ţ���Ϊ��֤����Һ�п��ܺ��е������Ƿ���ڣ����������ʵ�������֤������֤һ�����Ӽ��ɣ�
| ��Һ�п��ܺ��е����� | ���ʵ����֤��ʵ�鲽�衢����Ӧ�Ľ��ۣ� |
SO42- SO42- |
�������ᱵ��Һ�����г������и����� �������ᱵ��Һ�����г������и����� |
��������1���������������е��������ɵij���ֻ�����ᱵ��̼�ᱵ���з������
��2���ٸ����кͷ�Ӧ��ʵ�ʽ��з������
�ڸ��ݸ��ֽⷴӦ�����������Ӽ����ܻ����ϳɳ����������ˮ�������Ӳ��ܹ��森
��3���ٸ������е���Ϣ��Һ�����ԡ����ӹ�����������з������
�ڿ��ܺ��Т�Ba2+����SO42-����Ba2+��SO42-��Ϊ����֤����Һ�п��ܺ��е������Ƿ���ڣ����ʵ�������֤��
��4������ϡ�������������Ʒ�Ӧ�����Ȼ��ƺ�ˮ��������������Һ�����ʵ������������������Ӧ��������Һ�������Ȼ��Ƶ����������������������Һ�����ʵ������������ɣ�
��2���ٸ����кͷ�Ӧ��ʵ�ʽ��з������
�ڸ��ݸ��ֽⷴӦ�����������Ӽ����ܻ����ϳɳ����������ˮ�������Ӳ��ܹ��森
��3���ٸ������е���Ϣ��Һ�����ԡ����ӹ�����������з������
�ڿ��ܺ��Т�Ba2+����SO42-����Ba2+��SO42-��Ϊ����֤����Һ�п��ܺ��е������Ƿ���ڣ����ʵ�������֤��
��4������ϡ�������������Ʒ�Ӧ�����Ȼ��ƺ�ˮ��������������Һ�����ʵ������������������Ӧ��������Һ�������Ȼ��Ƶ����������������������Һ�����ʵ������������ɣ�
����⣺��1����������֮������ɵij���ֻ��̼�ᱵ���������ᱵ���������Ի�ѧ����ʽΪ��H2SO4+Ba��NO3��2=BaSO4��+2HNO3��K2CO3+Ba��NO3��2=BaCO3��+2KNO3���ʴ�Ϊ��H2SO4+Ba��OH��2=BaSO4��+2H2O��K2CO3+Ba��NO3��2=BaCO3��+2KNO3��
��2��������ϡ���������������Һ������ѧ��Ӧ���۹���ʾ��ͼ���û�ѧ��Ӧ��ʵ���������Ӻ���������������ˮ���ӣ����Ա�ʾΪH++OH-=H2O���ʴ�Ϊ�������Ӻ���������������ˮ���ӻ�H++OH-=H2O��
��3������Һ�У�Ba2+��SO42-���������ܽ�ϳ����ᱵ���������ܴ������森�ʴ�Ϊ��Ba2+ �� SO42-��
��3���ٸ������е���Ϣ��Һ�����ԣ�����Һ��һ����H+��ͨ�����ʵ��ܽ��Թ��ɿ�֪�����еļ��Ρ����Ρ������ζ�����ˮ������Һ��һ�����е�����Ϊ��H+��Na+��K+��NO3-��
�ʴ�Ϊ��K+��NO3-��H+��Na+��
�ڿ��ܺ��Т�Ba2+����SO42-����Ba2+��SO42-������ܺ���SO42-��ȡ������Һ�������Թ��У������������ᱵ��Һ�������ְ�ɫ��������˵��������Һ�к���SO42-����û�г��ְ�ɫ��������˵��������Һ�в�����SO42-��
�ʴ�Ϊ��SO42-���������ᱵ��Һ�����г������и����ӣ�
��4���⣺100g������������Ϊ16%������������Һ���ʵ�����Ϊ100g��16%=16g��
���Ȼ��Ƶ�����Ϊx��
NaOH+HCl=NaCl+H2O
40 58.5
16g x
=
X=23.4g
������Һ���ʵ���������=
��100%=11.7%��
��������Һ���ʵ���������Ϊ11.7%��
��2��������ϡ���������������Һ������ѧ��Ӧ���۹���ʾ��ͼ���û�ѧ��Ӧ��ʵ���������Ӻ���������������ˮ���ӣ����Ա�ʾΪH++OH-=H2O���ʴ�Ϊ�������Ӻ���������������ˮ���ӻ�H++OH-=H2O��
��3������Һ�У�Ba2+��SO42-���������ܽ�ϳ����ᱵ���������ܴ������森�ʴ�Ϊ��Ba2+ �� SO42-��
��3���ٸ������е���Ϣ��Һ�����ԣ�����Һ��һ����H+��ͨ�����ʵ��ܽ��Թ��ɿ�֪�����еļ��Ρ����Ρ������ζ�����ˮ������Һ��һ�����е�����Ϊ��H+��Na+��K+��NO3-��
�ʴ�Ϊ��K+��NO3-��H+��Na+��
�ڿ��ܺ��Т�Ba2+����SO42-����Ba2+��SO42-������ܺ���SO42-��ȡ������Һ�������Թ��У������������ᱵ��Һ�������ְ�ɫ��������˵��������Һ�к���SO42-����û�г��ְ�ɫ��������˵��������Һ�в�����SO42-��
�ʴ�Ϊ��SO42-���������ᱵ��Һ�����г������и����ӣ�
��4���⣺100g������������Ϊ16%������������Һ���ʵ�����Ϊ100g��16%=16g��
���Ȼ��Ƶ�����Ϊx��
NaOH+HCl=NaCl+H2O
40 58.5
16g x
| 40 |
| 58.5 |
| 16g |
| x |
������Һ���ʵ���������=
| 23.4g |
| 100g+100g |
��������Һ���ʵ���������Ϊ11.7%��
�����������ѶȲ��Ǻܴ��ۺ��Խ�ǿ�����⸴�ֽⷴӦ����������Ӧ��ʵ�ʡ��������ӵļ��鷽�������ո��ݻ�ѧ����ʽ�����������������ۺϼ�������ȷ�����Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
�ڿ����У�ͬѧ�������ᡢ���ᱵ���������ơ�̼����������ʵ���Һ�����и��ֽⷴӦ����������̽����
��1��ͨ���ж�����������Һ����֮���ܷ�����Ӧ��д�����з������������Ļ�ѧ����ʽ��
�г������ɵķ�Ӧ��______��
��2������ͼ1��HCl��NaOH��ˮ�н������ʾ��ͼ��ͼ2��ϡ������������Ʒ�Ӧ���۹���ʾ��ͼ��
��������������Ʒ�����Ӧ����ʵ����ʲô��______��
��ͨ�������ϵ�̽���ܷ��֣�����Һ�У�����H+��OH-���ܴ��������⣬______�����������ӷ��ţ�Ҳ���ܴ������森
��3�����������������ʵ���Һ���ʵ����������ʻ�ϲ����ˣ��õ�һ����ɫ��Һ��
�پ��ⶨ������Һ�����ԣ������Һ��һ�����е�������______��������д4�����ӷ��ţ�
��Ϊ��֤����Һ�п��ܺ��е������Ƿ���ڣ����������ʵ�������֤������֤һ�����Ӽ��ɣ�
| ��Һ�п��ܺ��е����� | ���ʵ����֤��ʵ�鲽�衢����Ӧ�Ľ��ۣ� |
| ______ | ______ |
�ڿ����У�ͬѧ�������ᡢ���ᱵ���������ơ�̼����������ʵ���Һ�����и��ֽⷴӦ����������̽����
��1��ͨ���ж�����������Һ����֮���ܷ�����Ӧ��д�����з������������Ļ�ѧ����ʽ��
�г������ɵķ�Ӧ��______��
��2������ͼ1��HCl��NaOH��ˮ�н������ʾ��ͼ��ͼ2��ϡ������������Ʒ�Ӧ���۹���ʾ��ͼ��

��������������Ʒ�����Ӧ����ʵ����ʲô��______��
��ͨ�������ϵ�̽���ܷ��֣�����Һ�У�����H+��OH-���ܴ��������⣬______�����������ӷ��ţ�Ҳ���ܴ������森
��3�����������������ʵ���Һ���ʵ����������ʻ�ϲ����ˣ��õ�һ����ɫ��Һ��
�پ��ⶨ������Һ�����ԣ������Һ��һ�����е�������______��������д4�����ӷ��ţ�
��Ϊ��֤����Һ�п��ܺ��е������Ƿ���ڣ����������ʵ�������֤������֤һ�����Ӽ��ɣ�
��4��100gϡ����ǡ�ø�100g������������Ϊ16%������������Һ��ȫ��Ӧ����������Һ�����ʵ�����������
��1��ͨ���ж�����������Һ����֮���ܷ�����Ӧ��д�����з������������Ļ�ѧ����ʽ��
�г������ɵķ�Ӧ��______��
��2������ͼ1��HCl��NaOH��ˮ�н������ʾ��ͼ��ͼ2��ϡ������������Ʒ�Ӧ���۹���ʾ��ͼ��

��������������Ʒ�����Ӧ����ʵ����ʲô��______��
��ͨ�������ϵ�̽���ܷ��֣�����Һ�У�����H+��OH-���ܴ��������⣬______�����������ӷ��ţ�Ҳ���ܴ������森
��3�����������������ʵ���Һ���ʵ����������ʻ�ϲ����ˣ��õ�һ����ɫ��Һ��
�پ��ⶨ������Һ�����ԣ������Һ��һ�����е�������______��������д4�����ӷ��ţ�
��Ϊ��֤����Һ�п��ܺ��е������Ƿ���ڣ����������ʵ�������֤������֤һ�����Ӽ��ɣ�
| ��Һ�п��ܺ��е����� | ���ʵ����֤��ʵ�鲽�衢����Ӧ�Ľ��ۣ� |
| ______ | ______ |
 ��2011?��³ľ�룩�ڸ�Ч��ϰ�����У�ͬѧ�����á����ᡢ���ᱵ���������ơ�̼��ء��������ʵ���Һ�ԡ����ֽⷴӦ������������������̽����
��2011?��³ľ�룩�ڸ�Ч��ϰ�����У�ͬѧ�����á����ᡢ���ᱵ���������ơ�̼��ء��������ʵ���Һ�ԡ����ֽⷴӦ������������������̽����