��Ŀ����
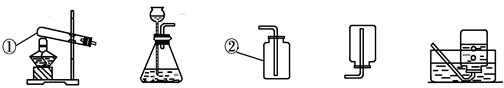
��11�֣���ͼ��ʵ������ȡ������̼��װ�ã�����������ѧ��ѧ֪ʶ�ش��������⣺
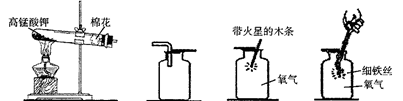
��1��ͼ�������ٵ������� �������ڵ������� ��
��2����ʵ��������� �ʹ���ʯ��Ӧ��ȡ������̼������Na2CO3�������ʯ��Ӧ��ԭ���� ��
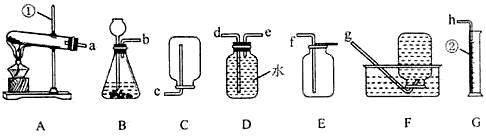
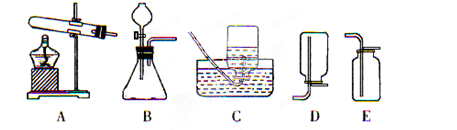
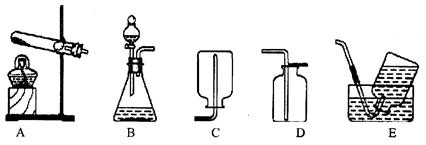
��3��С�����ʵ������ȡ������̼����ķ�Ӧԭ�������������װ�ã�������Ϊ��װ�â���װ�â���Ƚϣ�װ�� ���������һЩ��������
��Ϊ��̽��������̼��������ʣ�����������̼����ͨ�����ʯ��ˮ�У��۲쵽�а�ɫ���������÷�Ӧ�Ļ�ѧ����ʽΪ�� ��
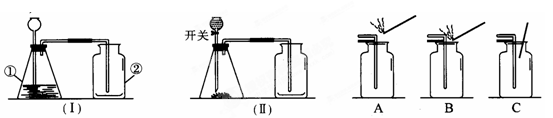
��4����ʵ��������� ���ռ�������̼���塣С���������������ͼ����װ�ã������ֲ�ͬ�ķ�����֤������̼�����Ƿ��ռ���������Ϊ��ѡ�� װ�ø�Ϊ������
��1��ͼ�������ٵ������� �������ڵ������� ��

��2����ʵ��������� �ʹ���ʯ��Ӧ��ȡ������̼������Na2CO3�������ʯ��Ӧ��ԭ���� ��
��3��С�����ʵ������ȡ������̼����ķ�Ӧԭ�������������װ�ã�������Ϊ��װ�â���װ�â���Ƚϣ�װ�� ���������һЩ��������
��Ϊ��̽��������̼��������ʣ�����������̼����ͨ�����ʯ��ˮ�У��۲쵽�а�ɫ���������÷�Ӧ�Ļ�ѧ����ʽΪ�� ��
��4����ʵ��������� ���ռ�������̼���塣С���������������ͼ����װ�ã������ֲ�ͬ�ķ�����֤������̼�����Ƿ��ռ���������Ϊ��ѡ�� װ�ø�Ϊ������
��1������©������ƿ
��2��ϡ���� ̼������ϡ���ᷴӦ����̫�죨�����Ʒ�Ӧ�Ľ��У�������ʵ������ȣ�
��3��������ʱ���Ƶμӵ��������ٶȣ� CO2 +Ca(OH)2==CaCO3�� +H2O
��4�������ſ����� B
��2��ϡ���� ̼������ϡ���ᷴӦ����̫�죨�����Ʒ�Ӧ�Ľ��У�������ʵ������ȣ�
��3��������ʱ���Ƶμӵ��������ٶȣ� CO2 +Ca(OH)2==CaCO3�� +H2O
��4�������ſ����� B
��1������ʵ���ҳ�����������ʶ�����⣻
��2����Ϥʵ������ȡ������̼�������õ�ҩƷ����������̼���ƴ������ʯ����Ӧԭ�ϵ�ԭ��
��3��̽��װ�â���װ�â�Ƚ���ȡ������̼��������ƣ�
��4�����ݶ�����̼�������ܶȴ��ڿ������ܶ���ѡ���ռ������Լ�������̼��ȼ��Ҳ��֧��ȼ�ս��н��
��2����Ϥʵ������ȡ������̼�������õ�ҩƷ����������̼���ƴ������ʯ����Ӧԭ�ϵ�ԭ��
��3��̽��װ�â���װ�â�Ƚ���ȡ������̼��������ƣ�
��4�����ݶ�����̼�������ܶȴ��ڿ������ܶ���ѡ���ռ������Լ�������̼��ȼ��Ҳ��֧��ȼ�ս��н��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ