��Ŀ����
26��������أ�K2Fe04���Ǿ�����ɫ�����ϸ�ᾧ��ĩ�����������Գ���������أ���һ�ּ����������������ۡ�ɱ����������һ������͡���Ч�Ķ��ˮ������������ĸ��������198���������ȶ��ģ������ֽ�Ϊ�����������������������������������Ϣ����ش��������⣺
�ٸ����������Ԫ�صĻ��ϼ�Ϊ
�ڸ�����ص�����������
�۱���������Ӧע��
�ٸ����������Ԫ�صĻ��ϼ�Ϊ
+6
���ڸ�����ص�����������
��ɫ�����ϸ�ᾧ��ĩ
���۱���������Ӧע��
���ȷ���������¸������������ȱܳ��ȣ�
���������ٸ����ڻ��������������ϼ۴�����Ϊ�㣬���н���⣻
�ڸ����������ʰ�������ɫ��״̬����ζ��ζ�����۵㡢�е㡢Ӳ�ȡ��ܶȡ������ԡ������ԡ���չ�ԡ��ܽ��ԡ��ӷ��Եȣ�������ɸѡ��Ϣ���ɣ�
�۸��ݸ���ĸ��������198���������ȶ��ģ������ֽ⣬����ѡ�淽����
�ڸ����������ʰ�������ɫ��״̬����ζ��ζ�����۵㡢�е㡢Ӳ�ȡ��ܶȡ������ԡ������ԡ���չ�ԡ��ܽ��ԡ��ӷ��Եȣ�������ɸѡ��Ϣ���ɣ�
�۸��ݸ���ĸ��������198���������ȶ��ģ������ֽ⣬����ѡ�淽����
����⣺�ٸ����ڻ��������������ϼ۴�����Ϊ�㣬�ɵø����������Ԫ�صĻ��ϼ�Ϊ����+1����2+x+��-2����4=0��
��x=+6���ʴ�Ϊ��+6��
�ڸ���������Ϣ��֪��������ص����������У���ɫ�����ϸ�ᾧ��ĩ���ʴ�Ϊ����ɫ�����ϸ�ᾧ��ĩ��
�۸��ݸ���ĸ��������198���������ȶ��ģ������ֽ⣬���Ա���������Ӧע�⣺���ȷ���������¸������������ȱܳ��ȣ����ʴ�Ϊ�����ȷ���������¸������������ȱܳ��ȣ���
��x=+6���ʴ�Ϊ��+6��
�ڸ���������Ϣ��֪��������ص����������У���ɫ�����ϸ�ᾧ��ĩ���ʴ�Ϊ����ɫ�����ϸ�ᾧ��ĩ��
�۸��ݸ���ĸ��������198���������ȶ��ģ������ֽ⣬���Ա���������Ӧע�⣺���ȷ���������¸������������ȱܳ��ȣ����ʴ�Ϊ�����ȷ���������¸������������ȱܳ��ȣ���
���������⿼������ڻ��������������ϼ۴�����Ϊ�����ָ��Ԫ�ػ��ϼ۵Ľ�������������������Ϣ���н����������

��ϰ��ϵ�д�
 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
�����Ŀ
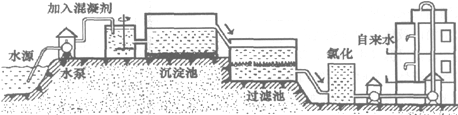
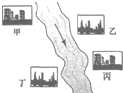 HCl�е�һ�֣�ij����С��Ժ�ˮ���ʱ���֣��״���ˮ����ɫ���Ҵ���ˮ�ʺ��ɫ��������ˮ�ɻ���壻�����������ݣ���ˮ���壮��ش�
HCl�е�һ�֣�ij����С��Ժ�ˮ���ʱ���֣��״���ˮ����ɫ���Ҵ���ˮ�ʺ��ɫ��������ˮ�ɻ���壻�����������ݣ���ˮ���壮��ش�