��Ŀ����
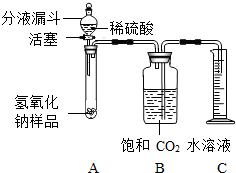 ijѧ��Ϊ�˲ⶨʵ������һƿ�治�ƶ����ֱ��ʵ�����������̼���Ƶ��������������������ͼ��ʾ��װ�ã�ͼ������̨����ȥ����ʵ����27�棬101kPa�½��У�ʵ�鲽�����£�
ijѧ��Ϊ�˲ⶨʵ������һƿ�治�ƶ����ֱ��ʵ�����������̼���Ƶ��������������������ͼ��ʾ��װ�ã�ͼ������̨����ȥ����ʵ����27�棬101kPa�½��У�ʵ�鲽�����£��ٰ�ͼ���Ӻ�װ�ã�������ƽȷ��ȡ����������Ʒ2g������A���Թ��ڣ���B�м���ƿ�ڵ��뱥�Ͷ�����̼ˮ��Һ��ƿ�����������Һ©���е���ϡ���ᣬ��������ϡ��������Թ������������رջ�������Ӧ��������Ͳ���ռ������Ͷ�����̼ˮ��Һ220mL����ش��������⣺
��1���ж��������Ʒ������ʵ�ʵ��������
��2����ʵ�鲽��ٺ͢�֮�䣬��ȱ��-ʵ�鲽�裬��ʵ�鲽����
��3��B�м���ƿʢװ�ı��Ͷ�����̼ˮ��Һ������ˮ���棬��������
��4���ж�ʵ�鲽����е����ϡ�����ѹ����ı�־��
��5������������Ʒ��̼���Ƶ���������Ϊ
��6��������װ�ò��ܲⶨ�Ѳ��ֱ��ʵ�����������Ʒ���������Ƶ�����������������
��������1���������������ʣ��������̼���ƣ���ô������ϡ����ʱ���������
��2����ʵ�������������룬���Ա��뱣֤װ�õ�����������
��3��������̼������ˮ
��4�����������Ʊ��ʣ�����������壬�����ٲ�������ʱ��˵����Ӧ���
��5�����ݶ�����̼�������������̼���Ƶ����������������������
��6�����������������տ����е�ˮ�ֶ�����
��2����ʵ�������������룬���Ա��뱣֤װ�õ�����������
��3��������̼������ˮ
��4�����������Ʊ��ʣ�����������壬�����ٲ�������ʱ��˵����Ӧ���
��5�����ݶ�����̼�������������̼���Ƶ����������������������
��6�����������������տ����е�ˮ�ֶ�����
����⣺��1�����������ڿ����������տ����е�ˮ�ֶ����⣬Ȼ���������еĶ�����̼��Ӧ�����ʣ������������������ʣ��������̼���ƣ���ô������ϡ����ʱ�����������̼���壬����A�л�ð�����ݣ�
�ʱ����Ϊ��A�������ݲ�����B���ռ�������/C���ռ���Һ�壩 ������е�CO2��Ӧ �ܷ�
��2����ʵ���������������룬����ʵ��ǰ������װ�õ������ԣ�
�ʱ����Ϊ�����װ��������
��3����Ӧ������Ҫ���ɶ�����̼����������̼���ˮ��Ӧ��������ˮ�����������ˮ�ᵼ��ʵ������ƫ�
�ʱ����Ϊ��������̼������ˮ
��4�����������Ʊ��ʣ�������ϡ����ʱ����������壬�����ٲ�������ʱ��˵����Ӧ��ϣ�
�ʱ����Ϊ������ϡ���ᣬA�в��������ݲ���
��5��47.7%
�⣺CO2��������m��CO2��=220mL��1000��1.8g/L=0.396g
����Ʒ��̼��������Ϊx
Na2CO3 +H2SO4�TNa2SO4+H2O+CO2��
106 44
X 0.396g
=
��X=0.954g
��Ʒ��̼���Ƶ���������Ϊ��
��100%=47.7%
��6�����������ڿ����������տ����е�ˮ�ֶ����⣬Ȼ��Ż�������еĶ�����̼��Ӧ�����ʣ�����û�в����ˮ�������������е����������Բ���������Ƶ�����������
�ʱ����Ϊ������������Ʒ�г���̼���ƣ�����ˮ
�ʱ����Ϊ��A�������ݲ�����B���ռ�������/C���ռ���Һ�壩 ������е�CO2��Ӧ �ܷ�
��2����ʵ���������������룬����ʵ��ǰ������װ�õ������ԣ�
�ʱ����Ϊ�����װ��������
��3����Ӧ������Ҫ���ɶ�����̼����������̼���ˮ��Ӧ��������ˮ�����������ˮ�ᵼ��ʵ������ƫ�
�ʱ����Ϊ��������̼������ˮ
��4�����������Ʊ��ʣ�������ϡ����ʱ����������壬�����ٲ�������ʱ��˵����Ӧ��ϣ�
�ʱ����Ϊ������ϡ���ᣬA�в��������ݲ���
��5��47.7%
�⣺CO2��������m��CO2��=220mL��1000��1.8g/L=0.396g
����Ʒ��̼��������Ϊx
Na2CO3 +H2SO4�TNa2SO4+H2O+CO2��
106 44
X 0.396g
| 106 |
| x |
| 44 |
| 0.396g |
��Ʒ��̼���Ƶ���������Ϊ��
| 0.954g |
| 2g |
��6�����������ڿ����������տ����е�ˮ�ֶ����⣬Ȼ��Ż�������еĶ�����̼��Ӧ�����ʣ�����û�в����ˮ�������������е����������Բ���������Ƶ�����������
�ʱ����Ϊ������������Ʒ�г���̼���ƣ�����ˮ
���������������������ơ�̼���Ƶ����ʣ�֪����������¶���ڿ������ױ��ʣ�������飬��ס��ѧ����ʽ��Na2CO3 +2HCl�T2NaCl+H2O+CO2�����������ĸ��ݻ�ѧ����ʽ���м��㣮

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
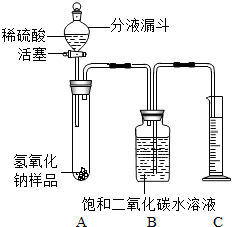 ijѧ��Ϊ�˲ⶨʵ������һƿ�治�ƶ����ֱ��ʵ�����������̼���Ƶ������������������ͼ��ʾ��װ�ã�ͼ������̨����ȥ����ʵ����27�棬101kPa�½��У�ʵ�鲽�����£�
ijѧ��Ϊ�˲ⶨʵ������һƿ�治�ƶ����ֱ��ʵ�����������̼���Ƶ������������������ͼ��ʾ��װ�ã�ͼ������̨����ȥ����ʵ����27�棬101kPa�½��У�ʵ�鲽�����£� ijѧ��Ϊ�˲ⶨʵ������һƿ�治�ƶ����ֱ��ʵ�����������̼���Ƶ������������������ͼ��ʾ��װ�ã�ͼ������̨�Ѿ���ȥ����ʵ����27�棬101kPa�½��У�ʵ�鲽�����£�
ijѧ��Ϊ�˲ⶨʵ������һƿ�治�ƶ����ֱ��ʵ�����������̼���Ƶ������������������ͼ��ʾ��װ�ã�ͼ������̨�Ѿ���ȥ����ʵ����27�棬101kPa�½��У�ʵ�鲽�����£�