��Ŀ����
ijѧ��Ϊ�˲ⶨʵ������һƿ�治�ƶ����ֱ��ʵ�����������̼���Ƶ������������������ͼ��ʾ��װ�ã�ͼ������̨����ȥ����ʵ����27�棬101kPa�½��У�ʵ�鲽�����£��ٰ�ͼ���Ӻ�װ�ã�
������ƽȷ��ȡ����������Ʒ2g������A���Թ��ڣ���B�м���ƿ�ڵ��뱥�Ͷ�����̼ˮ��Һ��ƿ������
�����Һ©���е���ϡ���ᣬ��������ϡ��������Թ������������رջ�������Ӧ��������Ͳ���ռ������Ͷ�����̼ˮ��Һ220mL��
��ش��������⣺
��1���ж��������Ʒ������ʵ�ʵ��������______���������Ʊ��ʵ�ԭ����______��
��2����ʵ�鲽��ٺ͢�֮�䣬��ȱ��һʵ�鲽�裬��ʵ�鲽����______��
��3��B�м���ƿʢװ�ı��Ͷ�����̼ˮ��Һ������ˮ���棬��������______��
��4���ж�ʵ�鲽����е����ϡ�����ѹ����ı�־��______��
��5������������Ʒ��̼���Ƶ���������Ϊ______����27�棬101kPaʱ������̼���ܶ�Ϊ1.8g/L��������̼����������ų��ı��Ͷ�����̼ˮ��Һ���������
��6��������װ�ò��ܲⶨ�Ѳ��ֱ��ʵ�����������Ʒ���������Ƶ�����������������______��

���𰸡���������1����������������ʻ�����̼���ƣ���ϡ�����ܲ���������̼���壮
��2����ʵ��ʱ���Ӻ�����һ��Ҫ���װ�õ������ԣ�
��3������ʵ��Ŀ�ġ�ͨ���������̼�����������ҩƷ��̼���Ƶĺ��������з�����
��4��֤��������ʵ�ʾ���֤��̼���Ʒ�Ӧ�꣮
��5����Ͳ���ռ������Ͷ�����̼ˮ��Һ220mL��Ϊ��Ӧ���ɵĶ�����̼��������ɴˣ����ɶ�����̼������Ϊ��220 mL/1000×1.8 g/L=0.396 g�����ݶ�����̼����������Ӧ�Ļ�ѧ����ʽ������̼���Ƶ����������ܵó���Ʒ��̼���Ƶ�����������
����⣺��1�����������Ǽ���ԺͿ����еĶ�����̼��Ӧ������̼���ƶ����ʣ����������ɵ�̼������ϡ��������ɶ�����̼���壮
��2��������������������ʵ���һ�㲽���ǣ�����װ�á�����װ�������ԡ�װҩƷ����ʵ�飮
��3�������ʵ��Ŀ����ͨ���������̼�����������ҩƷ��̼���Ƶĺ��������Ա��뱣֤������̼��ȷ�ԣ���Ϊ������̼��������ˮ�������ñ��͵Ķ�����̼ˮ��Һ�����Ա��������̼�ļ��٣�
��4��֤�������������֤��̼���Ʒ�Ӧ�꣬���Կ��Լ�����һ�����ᣬû�����ݲ�����
��5���⣺���ɶ�����̼������Ϊ��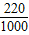 L×1.8 g/L=0.396 g
L×1.8 g/L=0.396 g
����Ʒ�к�̼���Ƶ�����Ϊx
Na2CO3+H2SO4=Na2SO4+CO2��+H2O
106 44
x 0.396g
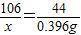 ����֮�ã�x=0.954g
����֮�ã�x=0.954g
����Ʒ��̼���Ƶ���������= ×100%=47.7%
×100%=47.7%
��6�����ʵ����������г�����̼�����⣬���������������տ����е�ˮ�֣���Ϊ����û�����ˮ��������Ҳ�Ͳ��ܼ����������Ƶ�����������
�ʴ�Ϊ����1��ϡ�����������������Ʒ�������ݲ����� �������������˿����еĶ�����̼������̼����
��2�����װ�õ�������
��3�����������̼�ܽ���ˮ�����ģ���ɲⶨ���ƫ��
��4����������ϡ���ᣬ���ٲ�������
��5��47.7%
��6�����ܲ�����ֱ��ʵ�����������������ˮ�֣�
������������һ���൱�ۺϵ����⣬���������������ڿ����б���Ϊ����㣬������������Ҫ�����ʵĻ�ѧ���ʽ����˿��飬ͬʱ�漰��װ�õ����ӡ����ݻ�ѧ����ʽ�ļ����ʵ����̵�̽���ȣ�����ѧ���Ƶ��о���
��2����ʵ��ʱ���Ӻ�����һ��Ҫ���װ�õ������ԣ�
��3������ʵ��Ŀ�ġ�ͨ���������̼�����������ҩƷ��̼���Ƶĺ��������з�����
��4��֤��������ʵ�ʾ���֤��̼���Ʒ�Ӧ�꣮
��5����Ͳ���ռ������Ͷ�����̼ˮ��Һ220mL��Ϊ��Ӧ���ɵĶ�����̼��������ɴˣ����ɶ�����̼������Ϊ��220 mL/1000×1.8 g/L=0.396 g�����ݶ�����̼����������Ӧ�Ļ�ѧ����ʽ������̼���Ƶ����������ܵó���Ʒ��̼���Ƶ�����������
����⣺��1�����������Ǽ���ԺͿ����еĶ�����̼��Ӧ������̼���ƶ����ʣ����������ɵ�̼������ϡ��������ɶ�����̼���壮
��2��������������������ʵ���һ�㲽���ǣ�����װ�á�����װ�������ԡ�װҩƷ����ʵ�飮
��3�������ʵ��Ŀ����ͨ���������̼�����������ҩƷ��̼���Ƶĺ��������Ա��뱣֤������̼��ȷ�ԣ���Ϊ������̼��������ˮ�������ñ��͵Ķ�����̼ˮ��Һ�����Ա��������̼�ļ��٣�
��4��֤�������������֤��̼���Ʒ�Ӧ�꣬���Կ��Լ�����һ�����ᣬû�����ݲ�����
��5���⣺���ɶ�����̼������Ϊ��
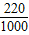 L×1.8 g/L=0.396 g
L×1.8 g/L=0.396 g����Ʒ�к�̼���Ƶ�����Ϊx
Na2CO3+H2SO4=Na2SO4+CO2��+H2O
106 44
x 0.396g
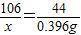 ����֮�ã�x=0.954g
����֮�ã�x=0.954g����Ʒ��̼���Ƶ���������=
 ×100%=47.7%
×100%=47.7%��6�����ʵ����������г�����̼�����⣬���������������տ����е�ˮ�֣���Ϊ����û�����ˮ��������Ҳ�Ͳ��ܼ����������Ƶ�����������
�ʴ�Ϊ����1��ϡ�����������������Ʒ�������ݲ����� �������������˿����еĶ�����̼������̼����
��2�����װ�õ�������
��3�����������̼�ܽ���ˮ�����ģ���ɲⶨ���ƫ��
��4����������ϡ���ᣬ���ٲ�������
��5��47.7%
��6�����ܲ�����ֱ��ʵ�����������������ˮ�֣�
������������һ���൱�ۺϵ����⣬���������������ڿ����б���Ϊ����㣬������������Ҫ�����ʵĻ�ѧ���ʽ����˿��飬ͬʱ�漰��װ�õ����ӡ����ݻ�ѧ����ʽ�ļ����ʵ����̵�̽���ȣ�����ѧ���Ƶ��о���

��ϰ��ϵ�д�
�����Ŀ
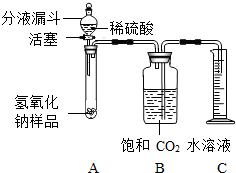
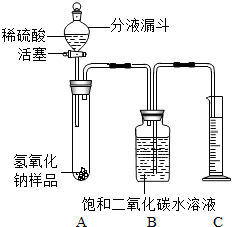 ijѧ��Ϊ�˲ⶨʵ������һƿ�治�ƶ����ֱ��ʵ�����������̼���Ƶ������������������ͼ��ʾ��װ�ã�ͼ������̨����ȥ����ʵ����27�棬101kPa�½��У�ʵ�鲽�����£�
ijѧ��Ϊ�˲ⶨʵ������һƿ�治�ƶ����ֱ��ʵ�����������̼���Ƶ������������������ͼ��ʾ��װ�ã�ͼ������̨����ȥ����ʵ����27�棬101kPa�½��У�ʵ�鲽�����£� ijѧ��Ϊ�˲ⶨʵ������һƿ�治�ƶ����ֱ��ʵ�����������̼���Ƶ������������������ͼ��ʾ��װ�ã�ͼ������̨�Ѿ���ȥ����ʵ����27�棬101kPa�½��У�ʵ�鲽�����£�
ijѧ��Ϊ�˲ⶨʵ������һƿ�治�ƶ����ֱ��ʵ�����������̼���Ƶ������������������ͼ��ʾ��װ�ã�ͼ������̨�Ѿ���ȥ����ʵ����27�棬101kPa�½��У�ʵ�鲽�����£�