��Ŀ����
�ᡢ�����������Ҫ�Ļ�����ռ����������Ƶ��׳ƣ�����һ����Ҫ�Ļ���ԭ�ϣ��㷺Ӧ���ڷ�������ֽ�ȹ�ҵ��
��1���ռ��ڹ�ҵ��ͨ���õ�ⱥ��ʳ��ˮ�ķ�����ȡ��������ռ��⣬����H2��Cl2����д����ⱥ��ʳ��ˮ�Ļ�ѧ����ʽ
��2���ռ��ڿ����з��û���ʣ��������һ��ʵ�飬���ܼ����ռ���ʵ�ͬʱ�����ܳ�ȥ���ʣ��û�ѧ����ʽ��ʾ
��3��������кܶͬ���ص㣬������ᶼ�ܹ������кͷ�Ӧ�������ᶼ��ʹ���ָʾ����ɫ�ȣ�������д����ͼ�����ɺ����ʷ���Ĺ�ͬ�㣨��дһ����
��4����ͬѧ�ù����ռ��ˮ����100g 18.5%��NaOH��Һ�����������������ռ���Һʱ�IJ�����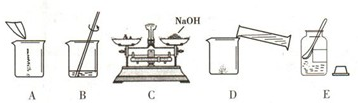
����ͼ��ʾ����ű�ʾ��ȷ���Ƹ���Һ�IJ���˳��Ϊ
����ͼC�����������Ϊ15g������Ķ���Ϊ3.5g����С���Ƶõ�������������ʵ��Ϊ
��5��ͬѧ����pH��ֽ�ⶨ�����Ƶ�����������Һ�����ȣ���ȷ�ķ�����
��6����8g��������Ϊ20%������������Һ��22gij����ǡ����ȫ�кͣ��Լ��㷴Ӧ��������Һ���ʵ�����������
��1���ռ��ڹ�ҵ��ͨ���õ�ⱥ��ʳ��ˮ�ķ�����ȡ��������ռ��⣬����H2��Cl2����д����ⱥ��ʳ��ˮ�Ļ�ѧ����ʽ
2NaCl+2H2O
2NaOH+Cl2��+H2��
| ||
2NaCl+2H2O
2NaOH+Cl2��+H2��
��
| ||
��2���ռ��ڿ����з��û���ʣ��������һ��ʵ�飬���ܼ����ռ���ʵ�ͬʱ�����ܳ�ȥ���ʣ��û�ѧ����ʽ��ʾ
Na2CO3+Ca��OH��2=CaCO3��+2NaOH
Na2CO3+Ca��OH��2=CaCO3��+2NaOH
����3��������кܶͬ���ص㣬������ᶼ�ܹ������кͷ�Ӧ�������ᶼ��ʹ���ָʾ����ɫ�ȣ�������д����ͼ�����ɺ����ʷ���Ĺ�ͬ�㣨��дһ����
��4����ͬѧ�ù����ռ��ˮ����100g 18.5%��NaOH��Һ�����������������ռ���Һʱ�IJ�����

����ͼ��ʾ����ű�ʾ��ȷ���Ƹ���Һ�IJ���˳��Ϊ
ECADB
ECADB
������ͼC�����������Ϊ15g������Ķ���Ϊ3.5g����С���Ƶõ�������������ʵ��Ϊ
11.5
11.5
g����5��ͬѧ����pH��ֽ�ⶨ�����Ƶ�����������Һ�����ȣ���ȷ�ķ�����
�ò������������Ƶ�����������Һ����pH��ֽ�ϣ���ͣ��ͱ���ɫ�Ƚϣ�������Һ��pH
�ò������������Ƶ�����������Һ����pH��ֽ�ϣ���ͣ��ͱ���ɫ�Ƚϣ�������Һ��pH
����6����8g��������Ϊ20%������������Һ��22gij����ǡ����ȫ�кͣ��Լ��㷴Ӧ��������Һ���ʵ�����������
��������ⱥ��ʳ��ˮ�������������ơ��������������������ɳ�������������ȷ����������ɣ��������ƺ����ᷴӦ�����Ȼ��ƺ�ˮ��������������ͨ��ָʾ������֤��֮�䷢���˻�ѧ��Ӧ�������������������ļ������ȷ����������Ʒ�����������Һʱ��Ҫ����ҩƷ���������������������ƫ�ٻ��ܼ�ƫ��Ĵ���������ᵼ�����ƵĽ��ƫ�ͣ�
����⣺��1����ⱥ��ʳ��ˮ�������������ơ����������������Ի�ѧ����ʽΪ��2NaCl+2H2O
2NaOH+H2��+Cl2����
��2�������������ƺͶ�����̼��Ӧ����̼���ƶ����ʣ�����������������������Һ���ܼ����ռ���ʵ�ͬʱ�����ܳ�ȥ���ʣ���ѧ����ʽ��ʾNa2CO3+Ca��OH��2=CaCO3��+2NaOH��
��3�����������ʿ�֪����ͼ�������϶�����Ԫ�أ���������ǿ��ǿ��и�ʴ�ԣ�
��4����ͬѧ�ù����ռ��ˮ����100g 18.5%��NaOH��Һ�����������������ռ���Һʱ�IJ���������ͼ��ʾ����ű�ʾ��ȷ���Ƹ���Һ�IJ���˳��Ϊ ECADB������ͼC�����������Ϊ15g������Ķ���Ϊ3.5g����С���Ƶõ�������������ʵ��Ϊ15g-3.5g=11.5g��
��5��ͬѧ����pH��ֽ�ⶨ�����Ƶ�����������Һ�����ȣ���ȷ�ķ������ò������������Ƶ�����������Һ����pH��ֽ�ϣ���ͣ��ͱ���ɫ�Ƚϣ�������Һ��pH��
��6���⣺�������Ȼ��Ƶ�����ΪX
HCl+NaOH�TNaCl+H2O
40 58.5
8g��20% X
X=2.34g
��Ӧ��������Һ���ʵ���������=
��100%=7.8%
�𣺷�Ӧ��������Һ���ʵ���������7.8%��
�ʴ�Ϊ����1��2NaCl+2H2O
2NaOH+Cl2��+H2����
��2��Na2CO3+Ca��OH��2=CaCO3��+2NaOH��
��3����ͼ�������϶�����Ԫ�أ���������ǿ��ǿ��и�ʴ�ԣ�
��4����ECADB����11.5��
��5���ò������������Ƶ�����������Һ����pH��ֽ�ϣ���ͣ��ͱ���ɫ�Ƚϣ�������Һ��pH��
��6��7.8%��
| ||
��2�������������ƺͶ�����̼��Ӧ����̼���ƶ����ʣ�����������������������Һ���ܼ����ռ���ʵ�ͬʱ�����ܳ�ȥ���ʣ���ѧ����ʽ��ʾNa2CO3+Ca��OH��2=CaCO3��+2NaOH��
��3�����������ʿ�֪����ͼ�������϶�����Ԫ�أ���������ǿ��ǿ��и�ʴ�ԣ�
��4����ͬѧ�ù����ռ��ˮ����100g 18.5%��NaOH��Һ�����������������ռ���Һʱ�IJ���������ͼ��ʾ����ű�ʾ��ȷ���Ƹ���Һ�IJ���˳��Ϊ ECADB������ͼC�����������Ϊ15g������Ķ���Ϊ3.5g����С���Ƶõ�������������ʵ��Ϊ15g-3.5g=11.5g��
��5��ͬѧ����pH��ֽ�ⶨ�����Ƶ�����������Һ�����ȣ���ȷ�ķ������ò������������Ƶ�����������Һ����pH��ֽ�ϣ���ͣ��ͱ���ɫ�Ƚϣ�������Һ��pH��
��6���⣺�������Ȼ��Ƶ�����ΪX
HCl+NaOH�TNaCl+H2O
40 58.5
8g��20% X
X=2.34g
��Ӧ��������Һ���ʵ���������=
| 2.34g |
| 8g+22g |
�𣺷�Ӧ��������Һ���ʵ���������7.8%��
�ʴ�Ϊ����1��2NaCl+2H2O
| ||
��2��Na2CO3+Ca��OH��2=CaCO3��+2NaOH��
��3����ͼ�������϶�����Ԫ�أ���������ǿ��ǿ��и�ʴ�ԣ�
��4����ECADB����11.5��
��5���ò������������Ƶ�����������Һ����pH��ֽ�ϣ���ͣ��ͱ���ɫ�Ƚϣ�������Һ��pH��
��6��7.8%��
���������⿼�����������Ƶ���ȡ�����йص����ʣ���Һ�����ƺ����������������йؼ��㣬��ɴ��⣬�����������е�֪ʶ����Һ���Ƶ�֪ʶ���У�

��ϰ��ϵ�д�
 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�
�����Ŀ
�ᡢ�����������Ҫ���ʣ�
��1��ʵ��������һƿ���ڷ��õ�Ũ���ᣮ�������������ͷ��������Ĺ�ϵ��ͼ������ˮ������������Ũ������������������仯��ԭ��______��

��2���۲�ͼƬ����С�Թ��ڵμ�ˮ�����������ƹ����ܽ��⣬���ɹ۲쵽��������______�����Ͳ����������ԭ��______��______��

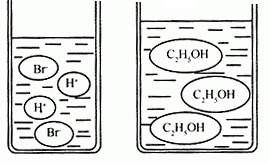
��3����ͼ�ֱ����廯�⣨HBr�����Ҵ���C2H5OH����ˮ�е���ʾ��ͼ�����������е��ᡢ��֪ʶ���ж��廯���ˮ��Һ��______���Ҵ���ˮ��Һ��______������ԡ��������ԡ����ԡ�����

��4����ʢ��10mLϡ���ᣨ���е�������ָʾ�������ձ��м�������������Һ����pH�Ʋⶨ��Һ��pH�������������£���������ش��������⣺
| ����NaOH��Һ�����/mL | 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 |
| �ձ�����Һ��pH | 1.1 | 1.2 | 1.4 | 1.6 | 2.5 | 7.0 | 11.0 | 12.0 |
�����μ�ָʾ������ɫ��̪��Һ��������������Һ�������Һ��ɫ�ޱ仯����ʱ��Һ�е�����һ���У��ѧʽ��______��������______��
��2009?��������ģ���ᡢ�����������Ҫ���ʣ�
��1��ʵ��������һƿ���ڷ��õ�Ũ���ᣮ�������������ͷ��������Ĺ�ϵ��ͼ������ˮ������������Ũ������������������仯��ԭ��
��2���۲�ͼƬ����С�Թ��ڵμ�ˮ�����������ƹ����ܽ��⣬���ɹ۲쵽�������ǣ����Ͳ����������ԭ��

��3����ͼ�ֱ����廯�⣨HBr�����Ҵ���C2H5OH����ˮ�е���ʾ��ͼ�����������е��ᡢ��֪ʶ���ж��廯���ˮ��Һ�ԣ��Ҵ���ˮ��Һ�ԣ�����ԡ��������ԡ����ԡ�����

��4����ʢ��10mLϡ���ᣨ���е�������ָʾ�������ձ��м�������������Һ����pH�Ʋⶨ��Һ��pH�������������£���������ش��������⣺
�����μӵ�ָʾ������ɫʯ����Һ������������������Һ�����Ϊ13mLʱ����Һ��ɫ��
�����μ�ָʾ������ɫ��̪��Һ��������������Һ�������Һ��ɫ�ޱ仯����ʱ��Һ�е�����һ���У��ѧʽ���������У�
��1��ʵ��������һƿ���ڷ��õ�Ũ���ᣮ�������������ͷ��������Ĺ�ϵ��ͼ������ˮ������������Ũ������������������仯��ԭ��

��2���۲�ͼƬ����С�Թ��ڵμ�ˮ�����������ƹ����ܽ��⣬���ɹ۲쵽�������ǣ����Ͳ����������ԭ��

��3����ͼ�ֱ����廯�⣨HBr�����Ҵ���C2H5OH����ˮ�е���ʾ��ͼ�����������е��ᡢ��֪ʶ���ж��廯���ˮ��Һ�ԣ��Ҵ���ˮ��Һ�ԣ�����ԡ��������ԡ����ԡ�����

��4����ʢ��10mLϡ���ᣨ���е�������ָʾ�������ձ��м�������������Һ����pH�Ʋⶨ��Һ��pH�������������£���������ش��������⣺
| ����NaOH��Һ�����/mL | 2 | 4 | 6 | 8 | 10 | 12 | 14 | |
| �ձ�����Һ��pH | 1.1 | 1.2 | 1.4 | 1.6 | 2.5 | 7.0 | 11.0 | 12.0 |
�����μ�ָʾ������ɫ��̪��Һ��������������Һ�������Һ��ɫ�ޱ仯����ʱ��Һ�е�����һ���У��ѧʽ���������У�