��Ŀ����
29���ᡢ�����������Ҫ���ʣ�
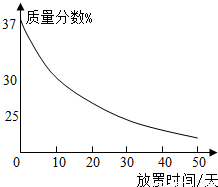
��1��ʵ��������һƿ���ڷ��õ�Ũ���ᣮ�������������ͷ��������Ĺ�ϵ��ͼ������ˮ������������Ũ������������������仯��ԭ��

��2���۲�ͼƬ����С�Թ��ڵμ�ˮ�����������ƹ����ܽ��⣬���ɹ۲쵽��������

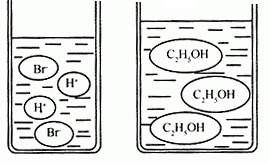
��3����ͼ�ֱ����廯�⣨HBr�����Ҵ���C2H5OH����ˮ�е���ʾ��ͼ�����������е��ᡢ��֪ʶ���ж��廯���ˮ��Һ��

��4����ʢ��10mLϡ���ᣨ���е�������ָʾ�������ձ��м�������������Һ����pH�Ʋⶨ��Һ��pH�������������£���������ش��������⣺

�����μӵ�ָʾ������ɫʯ����Һ������������������Һ�����Ϊ13mLʱ����Һ��
�����μ�ָʾ������ɫ��̪��Һ��������������Һ�������Һ��ɫ�ޱ仯����ʱ��Һ�е�����һ���У��ѧʽ��
��1��ʵ��������һƿ���ڷ��õ�Ũ���ᣮ�������������ͷ��������Ĺ�ϵ��ͼ������ˮ������������Ũ������������������仯��ԭ��
Ũ�����ӷ�
��
��2���۲�ͼƬ����С�Թ��ڵμ�ˮ�����������ƹ����ܽ��⣬���ɹ۲쵽��������
��īˮ��Һ���Ҹ��ڼס�����ʯ��ˮ�����
�����Ͳ����������ԭ����������ˮ��Ӧ����ʹ�Թ��ڵ�������������
���������Ƶ��ܽ�����¶ȵ����߶����ͣ�һ�����������ƹ�������
��
��3����ͼ�ֱ����廯�⣨HBr�����Ҵ���C2H5OH����ˮ�е���ʾ��ͼ�����������е��ᡢ��֪ʶ���ж��廯���ˮ��Һ��
����
���Ҵ���ˮ��Һ������
������ԡ��������ԡ����ԡ�����
��4����ʢ��10mLϡ���ᣨ���е�������ָʾ�������ձ��м�������������Һ����pH�Ʋⶨ��Һ��pH�������������£���������ش��������⣺

�����μӵ�ָʾ������ɫʯ����Һ������������������Һ�����Ϊ13mLʱ����Һ��
��
ɫ�������μ�ָʾ������ɫ��̪��Һ��������������Һ�������Һ��ɫ�ޱ仯����ʱ��Һ�е�����һ���У��ѧʽ��
NaCl
��������HCl
����������1������ͼ���������������������ʱ���ӳ��ı仯��������Ũ����Ļӷ��ԣ�����һ������н��ͣ�
��2��������������ˮ�ų������ȣ��ų���������ʹװ���ڿ����������ͣ�Ҳ��ʹ������Һʯ��ˮ��Һ�¶����ߣ�����������Ƶ��ܽ��ԣ�Ԥ�ƻ���ֵ�����˵��ԭ��
��3����Һ�к���H+����Һ�����ԣ���Һ�к�OH-����Һ��ʼ��ԣ���ͼ��ʾ��������Һ�����ӣ��ж�����Һ������ԣ�
��4���������ݱ����ж�������ϡ�����еμ�����������ҺpH�ı仯�����ݱ仯���ɣ��жϵ���������������Һ�����Ϊ13mLʱ����Һ������Լ���ɫʯ��ı�ɫ�����
�ڷ�̪�����������Һ��Ϊ��ɫ��ֻ������������Һ�ųʺ�ɫ�����ݷ�̪����ɫ���ƶ���Һ�з�Ӧ������ж���Һ�����ʣ�
��2��������������ˮ�ų������ȣ��ų���������ʹװ���ڿ����������ͣ�Ҳ��ʹ������Һʯ��ˮ��Һ�¶����ߣ�����������Ƶ��ܽ��ԣ�Ԥ�ƻ���ֵ�����˵��ԭ��
��3����Һ�к���H+����Һ�����ԣ���Һ�к�OH-����Һ��ʼ��ԣ���ͼ��ʾ��������Һ�����ӣ��ж�����Һ������ԣ�
��4���������ݱ����ж�������ϡ�����еμ�����������ҺpH�ı仯�����ݱ仯���ɣ��жϵ���������������Һ�����Ϊ13mLʱ����Һ������Լ���ɫʯ��ı�ɫ�����
�ڷ�̪�����������Һ��Ϊ��ɫ��ֻ������������Һ�ųʺ�ɫ�����ݷ�̪����ɫ���ƶ���Һ�з�Ӧ������ж���Һ�����ʣ�
����⣺��1��ͼʾ��������ʱ���ӳ����������������С�������Ũ��������ӷ������ʼ��Խ��ͣ���HCl���ϻӷ�����Һ������������С��
��2����������ˮ�ų������ȣ��Թ��ڿ����������ͣ�������֮��ͨ��U��������Һ����ָı䣬��īˮ��Һ���Ҹ��ڼף��ų�������ʹ���Թ���ʢ�ŵı�����Һ�¶����ߣ��������������ܽ�����¶����߶���С��������Һ�������¶��������壬�ɹ۲쵽ʯ��ˮ����ǣ�
��3����ͼʾ��֪���廯���ˮ��Һ�к���H+�������Һ�����ԣ����Ҵ���Һ�мȲ���H+Ҳ����OH-�������Һ�����ԣ�
��4������ɫʯ����������Һ�����ɫ�����ݱ������ݿ�֪��������10mL����������Һ��ǡ����ȫ��Ӧ����Һ�����ԣ���˵���13mL����������Һʱ�����������ƹ�����ʹ��Һ�ʼ��ԣ������ɫʯ�������ɫ��
�ڵ���һ��������������Һ��̪��Ϊ��ɫ�����ƶϴ�ʱ����Һ���ܺ���δ��Ӧ�������������ԣ���ǡ����ȫ��Ӧ�������ԣ���ˣ���ʱ��Һ��һ������Ӧ���ɵ��Ȼ��ƣ����ܺ���δ��ȫ��Ӧ�����
�ʴ�Ϊ��
��1��Ũ�����ӷ���
��2����īˮ��Һ���Ҹ��ڼס�����ʯ��ˮ����ǣ���������ˮ��Ӧ����ʹ�Թ��ڵ������������ͣ��������Ƶ��ܽ�����¶ȵ����߶����ͣ�һ�����������ƹ���������
��3�����ԣ����ԣ�
��4����������NaCl��HCl��
��2����������ˮ�ų������ȣ��Թ��ڿ����������ͣ�������֮��ͨ��U��������Һ����ָı䣬��īˮ��Һ���Ҹ��ڼף��ų�������ʹ���Թ���ʢ�ŵı�����Һ�¶����ߣ��������������ܽ�����¶����߶���С��������Һ�������¶��������壬�ɹ۲쵽ʯ��ˮ����ǣ�
��3����ͼʾ��֪���廯���ˮ��Һ�к���H+�������Һ�����ԣ����Ҵ���Һ�мȲ���H+Ҳ����OH-�������Һ�����ԣ�
��4������ɫʯ����������Һ�����ɫ�����ݱ������ݿ�֪��������10mL����������Һ��ǡ����ȫ��Ӧ����Һ�����ԣ���˵���13mL����������Һʱ�����������ƹ�����ʹ��Һ�ʼ��ԣ������ɫʯ�������ɫ��
�ڵ���һ��������������Һ��̪��Ϊ��ɫ�����ƶϴ�ʱ����Һ���ܺ���δ��Ӧ�������������ԣ���ǡ����ȫ��Ӧ�������ԣ���ˣ���ʱ��Һ��һ������Ӧ���ɵ��Ȼ��ƣ����ܺ���δ��ȫ��Ӧ�����
�ʴ�Ϊ��
��1��Ũ�����ӷ���
��2����īˮ��Һ���Ҹ��ڼס�����ʯ��ˮ����ǣ���������ˮ��Ӧ����ʹ�Թ��ڵ������������ͣ��������Ƶ��ܽ�����¶ȵ����߶����ͣ�һ�����������ƹ���������
��3�����ԣ����ԣ�
��4����������NaCl��HCl��
���������ݶ�ͼʾ��ʵ�����ݵķ�������ѧ���ƶ��������仯����������ǽ��������Ҫ�����ע�ģ�

��ϰ��ϵ�д�
�����Ŀ
�ᡢ�����������Ҫ���ʣ�
��1��ʵ��������һƿ���ڷ��õ�Ũ���ᣮ�������������ͷ��������Ĺ�ϵ��ͼ������ˮ������������Ũ������������������仯��ԭ��______��

��2���۲�ͼƬ����С�Թ��ڵμ�ˮ�����������ƹ����ܽ��⣬���ɹ۲쵽��������______�����Ͳ����������ԭ��______��______��

��3����ͼ�ֱ����廯�⣨HBr�����Ҵ���C2H5OH����ˮ�е���ʾ��ͼ�����������е��ᡢ��֪ʶ���ж��廯���ˮ��Һ��______���Ҵ���ˮ��Һ��______������ԡ��������ԡ����ԡ�����

��4����ʢ��10mLϡ���ᣨ���е�������ָʾ�������ձ��м�������������Һ����pH�Ʋⶨ��Һ��pH�������������£���������ش��������⣺
| ����NaOH��Һ�����/mL | 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 |
| �ձ�����Һ��pH | 1.1 | 1.2 | 1.4 | 1.6 | 2.5 | 7.0 | 11.0 | 12.0 |
�����μ�ָʾ������ɫ��̪��Һ��������������Һ�������Һ��ɫ�ޱ仯����ʱ��Һ�е�����һ���У��ѧʽ��______��������______��
��2009?��������ģ���ᡢ�����������Ҫ���ʣ�
��1��ʵ��������һƿ���ڷ��õ�Ũ���ᣮ�������������ͷ��������Ĺ�ϵ��ͼ������ˮ������������Ũ������������������仯��ԭ��
��2���۲�ͼƬ����С�Թ��ڵμ�ˮ�����������ƹ����ܽ��⣬���ɹ۲쵽�������ǣ����Ͳ����������ԭ��

��3����ͼ�ֱ����廯�⣨HBr�����Ҵ���C2H5OH����ˮ�е���ʾ��ͼ�����������е��ᡢ��֪ʶ���ж��廯���ˮ��Һ�ԣ��Ҵ���ˮ��Һ�ԣ�����ԡ��������ԡ����ԡ�����

��4����ʢ��10mLϡ���ᣨ���е�������ָʾ�������ձ��м�������������Һ����pH�Ʋⶨ��Һ��pH�������������£���������ش��������⣺
�����μӵ�ָʾ������ɫʯ����Һ������������������Һ�����Ϊ13mLʱ����Һ��ɫ��
�����μ�ָʾ������ɫ��̪��Һ��������������Һ�������Һ��ɫ�ޱ仯����ʱ��Һ�е�����һ���У��ѧʽ���������У�
��1��ʵ��������һƿ���ڷ��õ�Ũ���ᣮ�������������ͷ��������Ĺ�ϵ��ͼ������ˮ������������Ũ������������������仯��ԭ��

��2���۲�ͼƬ����С�Թ��ڵμ�ˮ�����������ƹ����ܽ��⣬���ɹ۲쵽�������ǣ����Ͳ����������ԭ��

��3����ͼ�ֱ����廯�⣨HBr�����Ҵ���C2H5OH����ˮ�е���ʾ��ͼ�����������е��ᡢ��֪ʶ���ж��廯���ˮ��Һ�ԣ��Ҵ���ˮ��Һ�ԣ�����ԡ��������ԡ����ԡ�����

��4����ʢ��10mLϡ���ᣨ���е�������ָʾ�������ձ��м�������������Һ����pH�Ʋⶨ��Һ��pH�������������£���������ش��������⣺
| ����NaOH��Һ�����/mL | 2 | 4 | 6 | 8 | 10 | 12 | 14 | |
| �ձ�����Һ��pH | 1.1 | 1.2 | 1.4 | 1.6 | 2.5 | 7.0 | 11.0 | 12.0 |
�����μ�ָʾ������ɫ��̪��Һ��������������Һ�������Һ��ɫ�ޱ仯����ʱ��Һ�е�����һ���У��ѧʽ���������У�