��Ŀ����
����Ŀ����(N2H4)�ǵ������γɵĻ����������ˮ����ҵ���������ط������£�ͬʱ�ø���Ʒʮˮ̼���ƣ������������£�
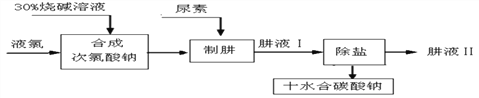
�����ϣ������صĻ�ѧʽΪCO(NH2)2�� ��Cl2+H2O==HCl+HClO��
�����¹��̵ķ�ӦΪCO(NH2)2+NaClO+2NaOH==N2H4+Na2CO3+NaCl+H2O��
����˵������ȷ����
A. ���ز�������ʯ�ҡ���ľ�ҵȼ������ʻ��ʹ�ã������ɰ�������ɷ�Ч��ʧ
B. Cl2��NaOH��Һ������Ӧ�Ļ�ѧ����ʽΪCl2+2NaOH==NaCl+NaClO+H2O
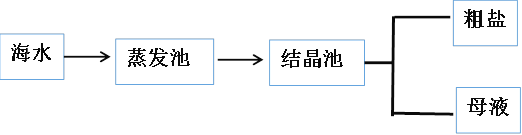
C. ��ĸҺ�з����ʮˮ��̼���ƾ��壬�ɲ�������Ũ������ȴ�ᾧ�����ˡ�ϴ�Ӹ���Ȳ���
D. �Ӻ�����Ӧ������������Ҫʹԭ����������ѣ��ϳɴ�������ʱͶ���Һ�����ռ������֮��Ϊ71 :80
���𰸡�AD
��������
A�������������ʷ�Ӧ�����ɰ�������ɷ�Ч��ʧ���������ز���笠�����������ʯ�ҡ���ľ�ҵȼ������ʻ��ʹ�ã������ɰ���������B������Cl2+H2O==HCl+HClO��Ȼ��HCl��HClO�������Ʒ�Ӧ����NaCl��NaClO��H2O�������ܷ�ӦΪ��Cl2+2NaOH==NaCl+NaClO+H2O����ȷ��C����ĸҺ�в�������Ũ������ȴ�ᾧ�����ˡ�ϴ�Ӹ���Ȳ�����Է����ʮˮ��̼���ƾ��壬��ȷ��D���� Cl2+2NaOH==NaCl+NaClO+H2OҺ�����ռ������֮��Ϊ71 :80���дӺ�����Ӧ�������������¹��̻���Ҫ�������ƣ���Ҫʹԭ����������ѣ��ϳɴ�������ʱͶ���Һ�����ռ������֮��ҪС��71 :80������ѡAD��

 ����ѧУ�ֲ����ܲ�ϵ�д�
����ѧУ�ֲ����ܲ�ϵ�д� �ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�ƸԺ���ȫ�����Ų��Ծ�ϵ�д�