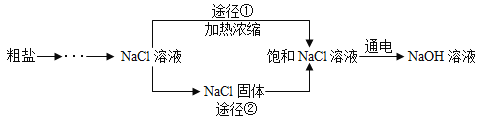
��Ŀ����
����Ŀ��ijͬѧ����ͼװ����ȡ���壬��ش�
![]() �ô���ʯ��ϡ������ȡ������̼����ķ���װ��Ӧѡ��______�������ռ�װ��Ӧѡ��______
�ô���ʯ��ϡ������ȡ������̼����ķ���װ��Ӧѡ��______�������ռ�װ��Ӧѡ��______![]() �����
�����![]() ���÷�Ӧ�Ļ�ѧ����ʽΪ______��
���÷�Ӧ�Ļ�ѧ����ʽΪ______��
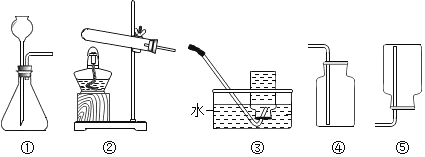
![]() ���װ��
���װ��![]() �����Եķ�����______��ʵ������װ��
�����Եķ�����______��ʵ������װ��![]() ��ȡ������ѧ����ʽ��______��
��ȡ������ѧ����ʽ��______��
![]() ��װ��
��װ��![]() ��ȡ����������ҩƷ��______ʱ�ռ����壬�����ռ����������л��н϶�Ŀ�����������______ʱ��˵�������Ѿ��ռ����ˡ�����ʵ��ʱӦ����______��______������ˮ���е�ˮ�������Թܶ�ʹ�Թ�ը�ѡ�
��ȡ����������ҩƷ��______ʱ�ռ����壬�����ռ����������л��н϶�Ŀ�����������______ʱ��˵�������Ѿ��ռ����ˡ�����ʵ��ʱӦ����______��______������ˮ���е�ˮ�������Թܶ�ʹ�Թ�ը�ѡ�
![]() ��װ��
��װ��![]() ��ȡ����ʱ���������Ƿ��Ѿ��ռ����ķ�����______����װ��
��ȡ����ʱ���������Ƿ��Ѿ��ռ����ķ�����______����װ��![]() ��ȡ������̼ʱ���������̼�Ƿ��Ѿ��ռ����ķ�����______��
��ȡ������̼ʱ���������̼�Ƿ��Ѿ��ռ����ķ�����______��
![]() ��װ��
��װ��![]() ������ʱ�ռ�����������dz��ɫ��ԭ����______��
������ʱ�ռ�����������dz��ɫ��ԭ����______��
���𰸡��� �� CaCO3+2HCl=CaCl2+H2O+CO2�� ��һ���������ڲ������ܿ��ϣ��������ϼ�һ��ֹˮ�У���©���м�ˮ��������ڳ���©�����γ�һ��ˮ�����ұ����ȶ�����װ�õ����������ã���֮��װ��©�� Zn+H2SO4=ZnSO4+H2�� ���ܿ���������������ð�� �����ݴӼ���ƿ������ð�� �Ƴ����� Ϩ��ƾ��� �������ǵ�ľ��ƽ���ڼ���ƿ�ڣ�ľ����ȼ��֤���������� ��һ��ȼ�ŵ�ľ��ƽ���ڼ���ƿ�ڣ�ľ��Ϩ��֤������ �Թܿ�û�з�����
��������
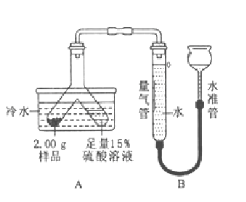
��1��ʵ������ȡCO2�����ڳ����£��ô���ʯ��ʯ��ʯ��ϡ������ȡ�ģ�̼��ƺ����ụ�ཻ���ɷ������Ȼ��ƺ�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2������˲���Ҫ���ȣ�������̼������ˮ���ܶȱȿ������ܶȴ����ֻ���������ſ������ռ���
��2�����װ�â������Եķ����ǣ���һ���������ڲ������ܿ��ϣ��������ϼ�һ��ֹˮ�У���©���м�ˮ��������ڳ���©�����γ�һ��ˮ�����ұ����ȶ�����װ�õ����������ã���֮��װ��©����п�����ᷴӦ��������п����������Ӧ�Ļ�ѧ����ʽΪ��Zn+H2SO4=ZnSO4+H2����
��3������ˮ���ռ��ϴ�������������ʱ���ǣ������ܿ���������������ð��ʱ����ˮ���ռ������ķ����ǣ��������ݴӼ���ƿ������ð��ʱ��֤�����ˣ�ʵ�����Ӧ���Ƴ����ܣ���Ϩ��ƾ��ƣ���ԭ���ǣ���ֹˮ������ʹ�Թ�ը�ѣ�
��4�����������������ǣ��������ǵ�ľ��ƽ���ڼ���ƿ�ڣ�ľ����ȼ��֤���������ˣ�������̼�����������ǣ���һ��ȼ�ŵ�ľ��ƽ���ڼ���ƿ�ڣ�ľ��Ϩ��֤�����ˣ�
��5����װ�â�������ʱ�ռ�����������dz��ɫ��ԭ���ǣ��Թܿ�û�з����š�

����Ŀ��ijͬѧ���α�Ҫ����CuSO4��Һ��NaOH��Һ��Ӧ��ʵ�飬����ɫ�������ɣ��Ըó������м��ȣ�һ�㿴����ɫ�������ɫ������ʱȴ������ɫ��������ɫ����ͬѧ�����쳣�����������Ȥ�������������������ʵ����������̽����
���������ϣ������Լ������ֽ�ɶ�Ӧ�Ľ���������
д������������ɫ�����Լ���ɫ�������ɫ�Ļ�ѧ��Ӧ����ʽ��___________��__________��
��������⣩������ɫ����û�г��ֺ�ɫ������ʲô�����йأ�
���������룩1.��CuSO4��Һ�����йأ�2.��NaOH��Һ�����й�
�����ʵ�飩��ѡ�Լ���1%��NaOH��Һ��20%NaOH��Һ��20%CuSO4��Һ��1%CuSO4��Һ
ʵ�鷽�� | ʵ������ | ʵ����� |
1.��2mL20%CuSO4��Һ�еμ�2��1%NaOH��Һ���ٽ����ó������ȡ� | �Ȳ�����ɫ���������Ⱥ� ______�� | ����1������ |
2._____���ٽ����ó������ȡ� | �Ȳ�����ɫ���������Ⱥ� _______�� | ����2���� |
���õ����ۣ�������ʵ���У�Ҫ�ܹ۲쵽��ɫ�������ɫ���ؼ���___________
����չ�뷴˼����һ���������Ͽ�֪������ɫ�������ܽ����������ͭ��С��ˮ����[��ѧʽΪCu4(OH)6SO4]���ڼ��Խ�ǿ�������������γɣ���������һ��ʱ�����ɫ������ɲ�����ɫ������д���÷�Ӧ����ʽ__________.