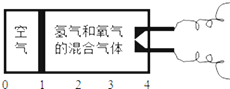
��Ŀ����
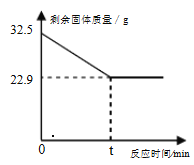
����Ŀ�����ó��ٽ�ˮ��������������·�壬�ܵõ���CuO��Cu2O��ɵĹ�����������������н�һ�������ɵ�����ͭ����(CuSO45 H2O)�����ʡ�������������ͼ��ʾ������˵����ȷ���� ( )
(��֪��Cu2O + H2SO4 CuSO4 + Cu + H2O)
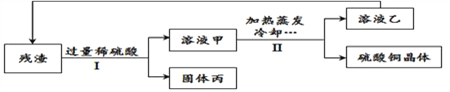
A. I�к����˲�����II�в������˲���
B. ��Һ����Һ��һ��Ϊ����ͭ�ı�����Һ
C. �����Ĵ��������У�H2SO4��CuSO4ʵ����ѭ������
D. ������I������22.4g����Ϊ6.4g����һ��ѭ�����ܵõ�����ͭ����50.0g
���𰸡�C
��������A������I����������õ��˹����Һ�壬����I����IJ����ж��й��ˣ�����B����Һ����Һ���о�������ͭ����Һ�ײ�һ��������ͭ�ı�����Һ����Һ��һ��������ͭ�ı�����Һ������C��ϡ������������ܽ�����������ͭ��Һ�������ٴνᾧ����������H2SO4��CuSO4ʵ����ѭ�����ã���ȷ��D��������22.4g����Ϊ6.4g��˵��Cu2O �� H2SO4��Ӧ����6.4g��ͭ���裺����6.4g��ͭ��ͬʱ���ɵ�����ͭ������Ϊx����Ҫ������Ϊy
Cu2O + H2SO4 CuSO4 + Cu + H2O
144 160 64
Y x 6.4g
![]() x=16g
x=16g
![]() y=14.4g
y=14.4g
CuO��Cu2O��ɵĹ��������CuO������=22.4g-14.4g=8g���裺8g��CuO��������ͭ������Ϊz
CuO+H2SO4=CuSO4+H2O
80 160
8g z
![]() z=16g
z=16g
������I�����ɵ�����ͭ������=16g+16g=32g��������ͭȫ��ת��Ϊ����ͭ���壬����ͭ���������=32g��![]() ��100%=50g�����ڼ�����������ȴ��������һ��������ͭ����Һ���У����Ծ���һ��ѭ�����ܵõ�����ͭ���������С��50.0g������ѡC��
��100%=50g�����ڼ�����������ȴ��������һ��������ͭ����Һ���У����Ծ���һ��ѭ�����ܵõ�����ͭ���������С��50.0g������ѡC��

����Ŀ����7�֣�ijͬѧ��ʵ���ҷ�����һƿ��ǩ��ȱ����ɫ��Һ�v��ͼ����ʾ�w��Ϊȷ�����е����ʣ�����Ʋ�����������̽�������ش��������⡣
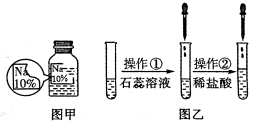
����������衿�����ʿ���ΪNaCl��NaOH��Na2CO3��NaHCO3�е�һ�֡�
�����ϲ��ġ������������ʵ������Ϣ���£�
���� | NaCl | NaOH | Na2CO3 | NaHCO3 |
�����µ��ܽ�ȣ�g | 36 | 109 | 21.5 | 9.6 |
������ijϡ��Һ��pH | 7 | 13 | 11 | 9 |
��̽�����̡�
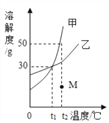
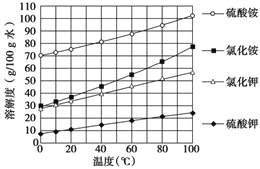
��ͼ����ʾ���ڲ����ٺ��ȷ�����ʲ���NaCl������ʵ������Ӧ�� ��
�ڽ��в�����ʱ����ɫ��ζ�õ�����������ɴ��ֿ��ų����������е� ��
��̽�����ۡ�
����Ϊ����Һ�е����ʿ������������������е� ������ж������� ��
��̽����˼��
��1��������̽����������ȷ�ģ������ڲ���������Ӧ�� ��д��ѧʽ����ʵ���Ҽ���������ʵ������������� ��
��2������ͬѧ�������е����ʻ�������Na2SO4������û��Na2SO4������������Ϣ��
�����������ʵ���������������жϸ���Һ�е������Ƿ���Na2SO4�����������ɣ� ��