��Ŀ����
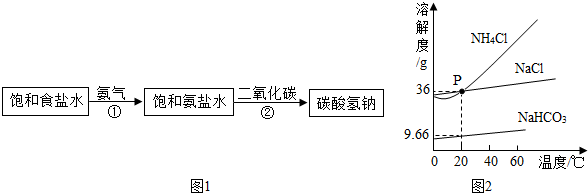
��ͼ1Ϊ�Ȼ��ơ�̼������ˮ�е��ܽ�����ߣ���ش��������⣺
��1��30��ʱ���Ȼ��Ƶ��ܽ�� �����������������=����̼���Ƶ��ܽ�ȣ�
��2��10��ʱ��10g̼���Ƽ��뵽50gˮ�в��Ͻ��裬�γɵ���Һ������ g��
��3��20��ʱ������ֻ��ʢ��100gˮ���ձ��У��ֱ�����Ȼ��ơ�̼�����������ʣ��������ܽ�Ϊֹ��������Һ�����ʵ����������Ĵ�С��ϵΪ�Ȼ��� ̼���ƣ����������������=������
��4����ͼ2��ʾ��20��ʱ����ʢ�б���̼������Һ��С�Թܷ���ʢˮ���ձ��У���ˮ�м���ij���ʺ��Թ����о�����������������ʿ����� ������ĸ��ţ���
A���������� B����ʯ�� C������� D��Ũ���ᣮ
���𰸡���������1�����������н���ĺ��忼�ǣ�
��2���ؼ�Ҫ���ݸ��¶�ʱ���ܽ�ȿ����������Ƿ�ȫ���ܽ⣻
��3�����ݱ�����Һ�����������ļ��㷽�����ǣ�
��4����������ˮ���Ⱥͷ���������ǣ�
����⣺
��1�������н����ʾ���¶�ʱ�������ʵ��ܽ����ȣ�����30��ʱ�Ȼ��ƺ�̼���������ʵ��ܽ����ȣ�
��2��10��ʱ��̼���Ƶ��ܽ��Ϊ10g��Ҳ����˵100gˮ������ܽ�10g̼���ƣ����Լ���10gֻ���ܽ�5g����Һ����Ϊ55g��
��3��������Һ���������������øù�ʽ���㣺 ×100%�����Ա�����Һ����������ȡ�����ܽ�ȵĴ�С����20��ʱ�Ȼ��Ƶ��ܽ�ȴ���̼���ƣ����Ա�����Һ����������Ҳ��
×100%�����Ա�����Һ����������ȡ�����ܽ�ȵĴ�С����20��ʱ�Ȼ��Ƶ��ܽ�ȴ���̼���ƣ����Ա�����Һ����������Ҳ��
��4���ɱ���̼������Һ��С�Թܷ���ʢˮ���ձ��У���ˮ�м���ij���ʺ��Թ����о���������˵���¶Ƚ����ˣ�Ҳ����˵����������ˮ�����ȵģ�����Ϊ����泥�
�ʴ�Ϊ����1���T����2��55����3��������4��C��
������ͨ���ش���֪�����ܽ�����߱�ʾ�����壬֪���˽���ĺ��壬������Һ�������������ļ��㹫ʽ����������ˮ���Ⱥͷ��������
��2���ؼ�Ҫ���ݸ��¶�ʱ���ܽ�ȿ����������Ƿ�ȫ���ܽ⣻
��3�����ݱ�����Һ�����������ļ��㷽�����ǣ�
��4����������ˮ���Ⱥͷ���������ǣ�
����⣺
��1�������н����ʾ���¶�ʱ�������ʵ��ܽ����ȣ�����30��ʱ�Ȼ��ƺ�̼���������ʵ��ܽ����ȣ�
��2��10��ʱ��̼���Ƶ��ܽ��Ϊ10g��Ҳ����˵100gˮ������ܽ�10g̼���ƣ����Լ���10gֻ���ܽ�5g����Һ����Ϊ55g��
��3��������Һ���������������øù�ʽ���㣺
 ×100%�����Ա�����Һ����������ȡ�����ܽ�ȵĴ�С����20��ʱ�Ȼ��Ƶ��ܽ�ȴ���̼���ƣ����Ա�����Һ����������Ҳ��
×100%�����Ա�����Һ����������ȡ�����ܽ�ȵĴ�С����20��ʱ�Ȼ��Ƶ��ܽ�ȴ���̼���ƣ����Ա�����Һ����������Ҳ����4���ɱ���̼������Һ��С�Թܷ���ʢˮ���ձ��У���ˮ�м���ij���ʺ��Թ����о���������˵���¶Ƚ����ˣ�Ҳ����˵����������ˮ�����ȵģ�����Ϊ����泥�
�ʴ�Ϊ����1���T����2��55����3��������4��C��
������ͨ���ش���֪�����ܽ�����߱�ʾ�����壬֪���˽���ĺ��壬������Һ�������������ļ��㹫ʽ����������ˮ���Ⱥͷ��������

��ϰ��ϵ�д�
 һ����������ϵ�д�
һ����������ϵ�д�
�����Ŀ
�����Ǹ���ı��⣬Ϊ�����ṩ�˱������Ȼ��Դ��
��1��ʳ�ú�����ȡ����ҪӪ������________��
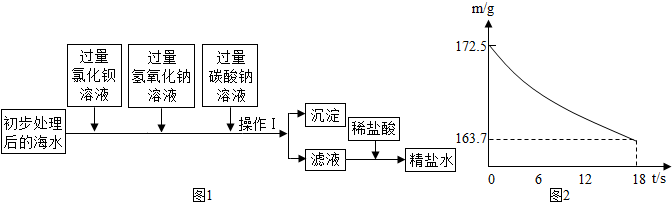
��2����ͼ1Ϊ��ˮ����װ�ã����õ���Դ��________����õ�����ˮ����________��
A������ B������� C�������
��3�����ú�ˮ����ȡ�������Ʒ���ú�ˮ��ʳ�Σ���ͨ����ˮɹ�εõ����Σ������к���������CaCl2��Na2SO4�����ʣ���ȥ�������ʣ��ȼӹ�����BaCl2��Һ��ȥ���ټӹ���________��Һ��ȥCaCl2������BaCl2�����ɵij�����________��ȥ��������________����pH���������ᾧ���Ƶô��Σ�
��4�����Ȼ��ƺ�̼����刺��Ʊ�̼�����ƺ��Ȼ�泥��÷�Ӧ��ѧ����ʽ�ɱ�ʾΪ��NaCl+NH4HCO3=NaHCO3+NH4Cl.20��ʱ����������ѧ����ʽ�з�Ӧ��������ȣ���100��ˮ�м���11.7��NaCl��15.8��NH4HCO3�������ϴ���Һ��������������Ϊ________g��
���ϣ�20��ʱ�������ʵ��ܽ�����£���������ͬʱ�ܽ���ˮ�и��Ե��ܽ�Ȳ��䣬
| ���� | NaCl | NH4CO3 | NH4Cl | NaHCO3 |
| �ܽ�� | 36.0 | 21.6 | 37.2 | 9.6 |
��д�������û����嵥�ʵĻ�ѧ����ʽ��________���÷�Ӧ��pH=3�����������½��У�����________�ⶨ��ӦҺ�����ȣ�
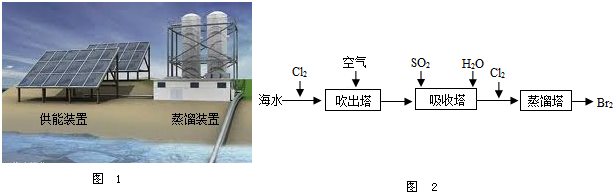
�ڴ�������ʹ������������һ��������������������з����ķ�Ӧ��Br2+SO2+2H2O?H2SO4+2HBr������������ͼ������ͨ��SO2��Ŀ����________��

�����Ǹ���ı��⣬Ϊ�����ṩ�˱������Ȼ��Դ��
��1��ʳ�ú�����ȡ����ҪӪ������ ��
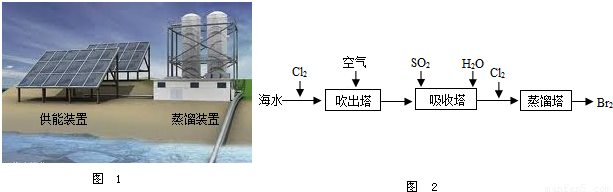
��2����ͼ1Ϊ��ˮ����װ�ã����õ���Դ�� ����õ�����ˮ���� ��
A������ B�������� C�������
��3�����ú�ˮ����ȡ�������Ʒ���ú�ˮ��ʳ�Σ���ͨ����ˮɹ�εõ����Σ������к���������CaCl2��Na2SO4�����ʣ���ȥ�������ʣ��ȼӹ�����BaCl2��Һ��ȥ���ټӹ��� ��Һ��ȥCaCl2������BaCl2�����ɵij����� ��ȥ�������� ����pH���������ᾧ���Ƶô��Σ�
��4�����Ȼ��ƺ�̼����刺��Ʊ�̼�����ƺ��Ȼ�泥��÷�Ӧ��ѧ����ʽ�ɱ�ʾΪ��NaCl+NH4HCO3=NaHCO3+NH4Cl.20��ʱ����������ѧ����ʽ�з�Ӧ��������ȣ���100��ˮ�м���11.7��NaCl��15.8��NH4HCO3�������ϴ���Һ��������������Ϊ g��
���ϣ�20��ʱ�������ʵ��ܽ�����£���������ͬʱ�ܽ���ˮ�и��Ե��ܽ�Ȳ��䣬
��5���Ӻ�ˮ����ȡ�峣�ô�������������������ˮ����廯���е����û����������ÿ�����ˮ���������壮����ʾ��ͼ��ͼ2��
��д�������û����嵥�ʵĻ�ѧ����ʽ�� ���÷�Ӧ��pH=3�����������½��У����� �ⶨ��ӦҺ�����ȣ�
�ڴ�������ʹ������������һ��������������������з����ķ�Ӧ��Br2+SO2+2H2O?H2SO4+2HBr������������ͼ������ͨ��SO2��Ŀ���� ��
��1��ʳ�ú�����ȡ����ҪӪ������ ��
��2����ͼ1Ϊ��ˮ����װ�ã����õ���Դ�� ����õ�����ˮ���� ��
A������ B�������� C�������
��3�����ú�ˮ����ȡ�������Ʒ���ú�ˮ��ʳ�Σ���ͨ����ˮɹ�εõ����Σ������к���������CaCl2��Na2SO4�����ʣ���ȥ�������ʣ��ȼӹ�����BaCl2��Һ��ȥ���ټӹ��� ��Һ��ȥCaCl2������BaCl2�����ɵij����� ��ȥ�������� ����pH���������ᾧ���Ƶô��Σ�
��4�����Ȼ��ƺ�̼����刺��Ʊ�̼�����ƺ��Ȼ�泥��÷�Ӧ��ѧ����ʽ�ɱ�ʾΪ��NaCl+NH4HCO3=NaHCO3+NH4Cl.20��ʱ����������ѧ����ʽ�з�Ӧ��������ȣ���100��ˮ�м���11.7��NaCl��15.8��NH4HCO3�������ϴ���Һ��������������Ϊ g��
���ϣ�20��ʱ�������ʵ��ܽ�����£���������ͬʱ�ܽ���ˮ�и��Ե��ܽ�Ȳ��䣬
| ���� | NaCl | NH4CO3 | NH4Cl | NaHCO3 |
| �ܽ�� | 36.0 | 21.6 | 37.2 | 9.6 |
��д�������û����嵥�ʵĻ�ѧ����ʽ�� ���÷�Ӧ��pH=3�����������½��У����� �ⶨ��ӦҺ�����ȣ�
�ڴ�������ʹ������������һ��������������������з����ķ�Ӧ��Br2+SO2+2H2O?H2SO4+2HBr������������ͼ������ͨ��SO2��Ŀ���� ��