��Ŀ����
��2013?��������ģ���ҹ���ѧ�Һ�°����ġ������Ƽ���������Ժ�ˮ��ɹ�Ρ��õ����Ȼ���Ϊԭ�ϣ�ͬʱ�Ƶ�NaHCO3��NH4Cl��Ʒ����̼�������Լ��ȼ��ֽ�õ������ͼ1����

��1��ͨ����Ӧ�ڿɵõ�̼�����ƣ���ѧ����ʽΪ��NaCl+NH4HCO3=NaHCO3+NH4Cl���÷�Ӧ����
��2��NaCl��NaHCO3��NH4Cl���ܽ��������ͼ2��
�ش��������⣺
��P��ĺ��壺
��̼�������ܽ�������ڴ���60��͡���ʧ���ˣ���˵��ԭ��
��20��ʱ����100gˮ�м���11.7gNaCl��15.8gNH4HCO3������Ӧ���Ƶ�NaHCO3�������ϴ���Һ���������������Ϊ

��1��ͨ����Ӧ�ڿɵõ�̼�����ƣ���ѧ����ʽΪ��NaCl+NH4HCO3=NaHCO3+NH4Cl���÷�Ӧ����
���ֽ�
���ֽ�
��Ӧ���ͣ���2��NaCl��NaHCO3��NH4Cl���ܽ��������ͼ2��
�ش��������⣺
��P��ĺ��壺
20��ʱNH4Cl��NaCl���ܽ�ȶ���36g
20��ʱNH4Cl��NaCl���ܽ�ȶ���36g
����̼�������ܽ�������ڴ���60��͡���ʧ���ˣ���˵��ԭ��
̼�����Ƽ����ֽ�õ�����
̼�����Ƽ����ֽ�õ�����
����20��ʱ����100gˮ�м���11.7gNaCl��15.8gNH4HCO3������Ӧ���Ƶ�NaHCO3�������ϴ���Һ���������������Ϊ
7.14
7.14
g����������1�����ݷ�Ӧ�����������ص������Ӧ���ͣ�
��2���ٸ����ܽ�����߽����ʾ���¶������ʵ��ܽ����ȷ�����
�ڸ�����Ϣ��̼�������Լ��ȼ��ֽ�õ����������
�۸���20��ʱ NaCl��NH4HCO3���ܽ�ȿ�֪��������ȫ�ܽ⣬�ɷ���ʽ������֮���������ϵ��֪NaCl��NH4HCO3ǡ����ȫ��Ӧ��������NaCl�������������ɵ�NaHCO3��NH4Cl����������ϸ��¶������ʵ��ܽ�ȷ���������������ʼ�������
��2���ٸ����ܽ�����߽����ʾ���¶������ʵ��ܽ����ȷ�����
�ڸ�����Ϣ��̼�������Լ��ȼ��ֽ�õ����������
�۸���20��ʱ NaCl��NH4HCO3���ܽ�ȿ�֪��������ȫ�ܽ⣬�ɷ���ʽ������֮���������ϵ��֪NaCl��NH4HCO3ǡ����ȫ��Ӧ��������NaCl�������������ɵ�NaHCO3��NH4Cl����������ϸ��¶������ʵ��ܽ�ȷ���������������ʼ�������
����⣺��1���÷�Ӧ�����ֻ�����������ɷ֣����������µĻ�����ķ�Ӧ���Ǹ��ֽⷴӦ��
��2����P��ĺ����ǣ�20��ʱNH4Cl��NaCl���ܽ�ȶ���36g��
����Ϊ��̼�������Լ��ȼ��ֽ�õ�������̼�������ܽ�������ڴ���60��͡���ʧ���ˣ�
�۽⣺��1�����ݻ�ѧ����ʽ��������ϵ����֪����NaCl��NH4HCO3ǡ����ȫ��Ӧ�������ɵ�NaHCO3������Ϊx��NH4Cl������Ϊy
NaCl+NH4HCO3�TNaHCO3+NH4Cl
58.5 79 84
11.7g x
=
x=16.8g
�������е��ܽ�ȱ�����֪����20��ʱ100gˮ���ܹ��ܽ�̼�����Ƶ�����Ϊ9.66g�����������ϴ���Һ��������NaHCO3���������Ϊ16.8g-9.66g=7.14g��
�ʴ�Ϊ����1�����ֽ⣻��2��20��ʱNH4Cl��NaCl���ܽ�ȶ���36g����3��̼�����Ƽ����ֽ�õ����
��4��7.14g��
��2����P��ĺ����ǣ�20��ʱNH4Cl��NaCl���ܽ�ȶ���36g��
����Ϊ��̼�������Լ��ȼ��ֽ�õ�������̼�������ܽ�������ڴ���60��͡���ʧ���ˣ�
�۽⣺��1�����ݻ�ѧ����ʽ��������ϵ����֪����NaCl��NH4HCO3ǡ����ȫ��Ӧ�������ɵ�NaHCO3������Ϊx��NH4Cl������Ϊy
NaCl+NH4HCO3�TNaHCO3+NH4Cl
58.5 79 84
11.7g x
| 58.5g |
| 11.7g |
| 84 |
| x |
�������е��ܽ�ȱ�����֪����20��ʱ100gˮ���ܹ��ܽ�̼�����Ƶ�����Ϊ9.66g�����������ϴ���Һ��������NaHCO3���������Ϊ16.8g-9.66g=7.14g��
�ʴ�Ϊ����1�����ֽ⣻��2��20��ʱNH4Cl��NaCl���ܽ�ȶ���36g����3��̼�����Ƽ����ֽ�õ����
��4��7.14g��
���������⿼����ѧ�����������������龰��ͼ����Ϣ�������ѧ�ܽ�ȡ�����ʽ��������֪ʶ�ͼ��ܣ�ϸ�ĵ�̽����������������ĿҪ��������������������������

��ϰ��ϵ�д�
 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�
�����Ŀ
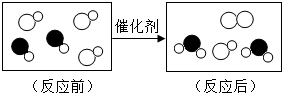 ��2013?��������ģ����β����ת���������Խ�����β�����ж�����ת��Ϊ�����壬��ͼ��ʾ������һ����ѧ ��Ӧ����ʾ��ͼ�����в�ͬ��Բ�������ͬ��ԭ�ӣ���ͼ�л����Ϣ����ȷ���ǣ�������
��2013?��������ģ����β����ת���������Խ�����β�����ж�����ת��Ϊ�����壬��ͼ��ʾ������һ����ѧ ��Ӧ����ʾ��ͼ�����в�ͬ��Բ�������ͬ��ԭ�ӣ���ͼ�л����Ϣ����ȷ���ǣ�������