��Ŀ����
ij��ѧ������ȤС�����о�����˿��������ȼ�յ�Ӱ�����ء�ʵ��ʱ����Ҫ��ȡһ��������������ͼ�Ǹ�С��ס��ҡ���������λͬѧ��Ƶļ�����������װ�ü�ѡ�õ��Լ����ش��������⣺
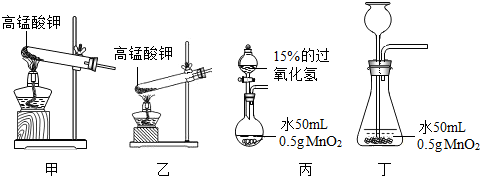
��1��д����ͬѧ��ȡ�����Ļ�ѧ��Ӧ����ʽ��______��
��2����װ�ú�ѡ�õ��Լ��������д������______����ס������ҡ���������������������
��3��д����ͬѧ����װ����һ�ֲ������������ƣ�______�����ܺ���Ƥ�����⣩��
��4����ͬѧ��ȡ���ռ�һƿ����������˿��������ȼ�յ�ʵ�飬���δ���������������������ΪӰ���ͬѧʵ��ʧ�ܵĿ���������______��д��һ�����ɣ���
��5����ͬѧʵ��ʱȡ����15%�Ĺ���������Һ20mL�����ܶ�Ϊ1g/mL��������ȡ��Һ�к�H2O2______g��ʵ���Ҫ����MnO2��Ӧ��ȡ��ʵ�����������______��

��1��д����ͬѧ��ȡ�����Ļ�ѧ��Ӧ����ʽ��______��
��2����װ�ú�ѡ�õ��Լ��������д������______����ס������ҡ���������������������
��3��д����ͬѧ����װ����һ�ֲ������������ƣ�______�����ܺ���Ƥ�����⣩��
��4����ͬѧ��ȡ���ռ�һƿ����������˿��������ȼ�յ�ʵ�飬���δ���������������������ΪӰ���ͬѧʵ��ʧ�ܵĿ���������______��д��һ�����ɣ���
��5����ͬѧʵ��ʱȡ����15%�Ĺ���������Һ20mL�����ܶ�Ϊ1g/mL��������ȡ��Һ�к�H2O2______g��ʵ���Ҫ����MnO2��Ӧ��ȡ��ʵ�����������______��
��1����������������Ļ�ѧ����ʽΪ2KMnO4
K2MnO4+MnO2+O2��
��2����װ���Թܿ�������б����ʹˮ�鵹�����ȵ��Թܵ�ʹ�Թ�ը�ѣ���װ�����õ�ҩƷֻ��ˮ��û��˫��ˮ�����ҡ����д���
��3�������������˵�����г���©������ƿ
��4����˿��������ȼ��ʵ������Ҫ����˿�ϵ����ĥ����Ȼ������˿ĩ��Ӧϵ��һ�������ȼ��˿������Ũ��Ҫ�ߣ�����ʵ����ܳɹ���
��5�������������������ļ��㹫ʽ��֪���������T��Һ��������������������������Һ��˫��ˮ������=20mL��1g/mL��15%=3g���������̲�����ˮ�����Կ��ù��˲������ն������̣�
�ʴ�Ϊ����1��2KMnO4
K2MnO4+MnO2+O2��
��2���ҡ���
��3������©������ƿ
��4����˿����
��5��3g������
| ||
��2����װ���Թܿ�������б����ʹˮ�鵹�����ȵ��Թܵ�ʹ�Թ�ը�ѣ���װ�����õ�ҩƷֻ��ˮ��û��˫��ˮ�����ҡ����д���
��3�������������˵�����г���©������ƿ
��4����˿��������ȼ��ʵ������Ҫ����˿�ϵ����ĥ����Ȼ������˿ĩ��Ӧϵ��һ�������ȼ��˿������Ũ��Ҫ�ߣ�����ʵ����ܳɹ���
��5�������������������ļ��㹫ʽ��֪���������T��Һ��������������������������Һ��˫��ˮ������=20mL��1g/mL��15%=3g���������̲�����ˮ�����Կ��ù��˲������ն������̣�
�ʴ�Ϊ����1��2KMnO4
| ||
��2���ҡ���
��3������©������ƿ
��4����˿����
��5��3g������

��ϰ��ϵ�д�
 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д�
�����Ŀ
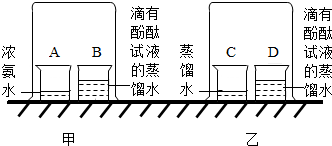
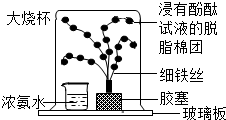

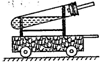 20������һ��ij�ο���������һ����Ȥ�ı�������˭�����ֳ�һ�����еľ�����Զ����������ѧ��ȤС����������ͼ��ʾ��һ��С�����������Թ��м��������ּ����������ӣ�ͨ���������������ʱ�ķ�������ʹС����ǰ�˶��ˣ�����ѡ����Լ�����п�ۣ���ͭ�ۣ������ۣ���10%��ϡ���ᣬ��10%��ϡ���ᣩ
20������һ��ij�ο���������һ����Ȥ�ı�������˭�����ֳ�һ�����еľ�����Զ����������ѧ��ȤС����������ͼ��ʾ��һ��С�����������Թ��м��������ּ����������ӣ�ͨ���������������ʱ�ķ�������ʹС����ǰ�˶��ˣ�����ѡ����Լ�����п�ۣ���ͭ�ۣ������ۣ���10%��ϡ���ᣬ��10%��ϡ���ᣩ 15��ij��ѧ������ȤС�飬��չ������������
15��ij��ѧ������ȤС�飬��չ������������ ʵ���������Һ��pH�仯��������ͼ��ʾ��
ʵ���������Һ��pH�仯��������ͼ��ʾ��
