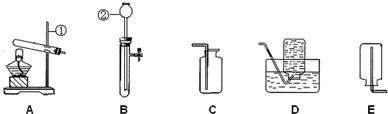��Ŀ����
ij��ѧ������Ȥ�С�������һϵ�е�ʵ��̽�����밴Ҫ��ش�
��1��д��ͼ�б��Т١��ڵ��������ƣ���
��2��Сǿ���ø��������ȡ��������Ӧѡ������װ���е�
��3��ʵ�����ռ���������D��Eװ�õ�ԭ��ֱ���
��4�������ǵ���Ҫ�ɷ���̼��ƣ�Ϊ�˲ⶨij��������̼��Ƶ���������������Ȥ�С���СȺͬѧ����������ʵ�飺��������ϴ�����ɺ����ȡ10g�����ձ��Ȼ�����ձ��м���������ϡ����90g����ַ�Ӧ�Ƶ÷�Ӧʣ����Ϊ97.14g���������������ʲ������ᷴӦ��
�ٲ���������̼����
�ڼ���ü�������̼��Ƶ�����������
��������1����Ϥ�����������˽����ƣ�
��2������ʵ�����ø�������������IJ��������ע�����գ�
��3���ռ������װ��Ҫ����������ܶȺ�ˮ���Կ��ǣ�
��4���ٸ��������غ㶨�ɿ�֪�����ɶ�����̼�����������10g����Ƥ��90gϡ�������������ȥʣ������97.14g��������
�ڸ��ݼ���Ƥ��ϡ����Ļ�ѧ��Ӧʽ�͢����еõ��Ķ�����̼�����з��̼��㼴�ɣ�
��2������ʵ�����ø�������������IJ��������ע�����գ�
��3���ռ������װ��Ҫ����������ܶȺ�ˮ���Կ��ǣ�
��4���ٸ��������غ㶨�ɿ�֪�����ɶ�����̼�����������10g����Ƥ��90gϡ�������������ȥʣ������97.14g��������
�ڸ��ݼ���Ƥ��ϡ����Ļ�ѧ��Ӧʽ�͢����еõ��Ķ�����̼�����з��̼��㼴�ɣ�
����⣺��1��ͼ��������������̨�����dz���©�������ǻ�ѧʵ���еij���������
�ʴ�Ϊ������̨������©����
��2���ø��������ȡ�������ǹ���ļ��ȷ�Ӧ�����Կ�ѡ��װ��A��Ϊ����װ�ã������������ܶȴ��ڿ������ܶȣ�����������ˮ�����Կ�����װ��C�����ſ������ռ���Ҳ����װ��D��ˮ���ռ���Aװ�õ��Թܿ�ȱ�ٷ�ֹ������ؽ��뵼�ܵ�һ�������˷�Ӧ�Ļ�ѧ����ʽ�ǣ�2KMnO4
K2MnO4+MnO2+O2����
�ʴ�Ϊ��AC��AD��һ������2KMnO4
K2MnO4+MnO2+O2����
��3���������ܶ�С�ڿ������ܶȣ�����������ˮ�����������ſ���������ˮ���ռ���
�ʴ�Ϊ������������ˮ���ܶ�С�ڿ�����
��4����10g����Ƥ��90gϡ���ᣬʣ������97.14g�����������غ㶨�ɿ�֪�������Ķ�����̼�����ǣ�10+90-97.14=2.86g
�ʴ�Ϊ��2.86g��
�ڽ⣺��ʯ��ʯ��Ʒ��CaCO3������ΪX
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
X 2.86
=
X=6.5g
��������Ʒ��CaCO3����������Ϊ��
��100%�T65%
�𣺼�������CaCO3����������Ϊ65%��
�ʴ�Ϊ������̨������©����
��2���ø��������ȡ�������ǹ���ļ��ȷ�Ӧ�����Կ�ѡ��װ��A��Ϊ����װ�ã������������ܶȴ��ڿ������ܶȣ�����������ˮ�����Կ�����װ��C�����ſ������ռ���Ҳ����װ��D��ˮ���ռ���Aװ�õ��Թܿ�ȱ�ٷ�ֹ������ؽ��뵼�ܵ�һ�������˷�Ӧ�Ļ�ѧ����ʽ�ǣ�2KMnO4
| ||
�ʴ�Ϊ��AC��AD��һ������2KMnO4
| ||
��3���������ܶ�С�ڿ������ܶȣ�����������ˮ�����������ſ���������ˮ���ռ���
�ʴ�Ϊ������������ˮ���ܶ�С�ڿ�����
��4����10g����Ƥ��90gϡ���ᣬʣ������97.14g�����������غ㶨�ɿ�֪�������Ķ�����̼�����ǣ�10+90-97.14=2.86g
�ʴ�Ϊ��2.86g��
�ڽ⣺��ʯ��ʯ��Ʒ��CaCO3������ΪX
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
X 2.86
| 100 |
| X |
| 44 |
| 2.86 |
X=6.5g
��������Ʒ��CaCO3����������Ϊ��
| 6.5 |
| 10 |
�𣺼�������CaCO3����������Ϊ65%��
������ʵ��װ�õ�ѡ��Ҫ����Ӧ���״̬�ͷ�Ӧ������������ռ�װ��Ҫ���ռ�������ܶȺ���ˮ�ԣ��ⶼ�ǻ�ѧʵ���г������⣬ͬѧ��һ��Ҫ���壮

��ϰ��ϵ�д�
 ͬ������ϵ�д�
ͬ������ϵ�д�
�����Ŀ
 15��ij��ѧ������ȤС�飬��չ������������
15��ij��ѧ������ȤС�飬��չ������������ ʵ���������Һ��pH�仯��������ͼ��ʾ��
ʵ���������Һ��pH�仯��������ͼ��ʾ��