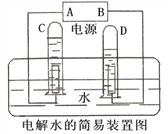
��Ŀ����
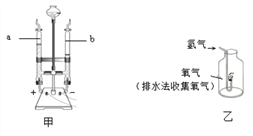
����Ŀ��Ϊ�ⶨ�����������ĺ������ס�������ͬѧ����ͼ��ʾ��װ�ã�������ͼ��ʾ��װ�÷ֱ������ʵ��̽����
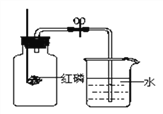
(1)��ͬѧ����ȼ������������ƿ�в�����ƿ����������ȼ��ֹͣ����ȴ��ֹˮ�У��۲쵽����ƿ������ˮ�����Լռ����ƿ�ݻ���_______��
(2)��ͬѧ����̼������ף��������ʵ�飬���ּ���ƿ�ڲ�û������ˮ����ԭ����_____________��
(3)��ͬѧ��������ͼ��ʾ��װ�òⶨ�����������ĺ������õ������ͬѧ��ͬ��ʵ�����������п��������ڵ�ˮ�����½���������������ѧ֪ʶ������һ����_______________________��
(4)����ȼ�յĻ�ѧʽ����ʽ��__________________________________��
���𰸡� 1/5 ̼ȼ���������Ƕ�����̼���壬ƿ����ѹ���� ��Ϊ��Ӧʱ�ų��������ȣ������˶����ʼӿ죬ʹ������ѹ����������ˮ���½�������Ӧ�������¶Ƚ���ʱ�����������μ��˷�Ӧ��������ѹǿ��С������ˮ�������� P+O2![]() P2O5
P2O5
����������1����Ϊ����ȼ�����Ŀ����е�����������Լռ���������1/5���ʼ���ƿ������ˮ�����Լռ����ƿ�ݻ���1/5��
��2��������������ĺ�����Ҫ�Ǹ�������ȼ�����Ŀ����е����������ѹ��С����С�����������������������������в��������壬��̼������ף�̼ȼ���������Ƕ�����̼���壬ƿ����ѹ���䣬�ʲ������ˮ��
��3��������������Ӧ�ų��������ȣ���ʹ�����˶����ʼӿ죬����������ͣ�ʹ������ѹ����������ˮ���½��������ڿ�����ȼ�ջ����Ŀ����е��������������������������ԼΪ1/5����ȴ��������������٣����ٵľ��ǿ��������������������ˮ����������
��4������ȼ���������������ף���ѧʽ����ʽ��P+O2![]() P2O5��
P2O5��

����Ŀ��ˮ��������Ϥ�����ʡ�
��Ϊ�˲ⶨˮ����ɣ���������̽����
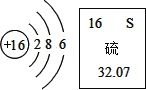
���������ˮʱ�������ڵ�������____________��b�������ɵ�������______________������a���ڲ�������ķ�����___________������������������___________����ʵ��˵��ˮ�����⡢��Ԫ����ɵ�����������__________��
���ˮʱ��������������������ʹ���ˮ��Ӧ�����У����������Ʊ����������ͻ�ѧ���ʲ������仯����ˮ���װ����ʢ��һ����������������Һ��ͨ��һ��ʱ�������16g������������Һ���������Ƶ����������� 2.7%��Ϊ3.0%���Լ���
��.�μӷ�Ӧ��ˮ�����ʵ���____________mol(���ݻ�ѧ����ʽ��ʽ����)��
��.���ǰ����������Һ������Ϊ________________g��
�ҷ������÷�����ʵ��Ŀ����____________________��������������ȼ�գ���Ӧ�Ļ�ѧ����ʽ��_________________������__________����ʵ���ܷ�ﵽʵ��Ŀ�ģ�����������___________��
���±���KNO3 ��NaCl�ڲ�ͬ�¶��µIJ����ܽ�����ݡ�
�¶�(��) | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 |
KNO3 | 13.3 | 20.9 | 32.0 | 45.8 | 64.0 | 85.5 | 110.0 | 138.0 | 169.0 | 202.0 | 246.0 |
NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 | 37.8 | 38.4 | 39.0 | 39.8 |
��. �������������ܽ�ȵı仯���¶�Ӱ���С����___________��
��. ��40��ʱ����40gKNO3�ܽ���50gˮ�У��γ���Һ��������_______g����������60�棬�γɵ���ҺΪ________(����������������������)��Һ������Һ��������������Ϊ_______��
��.���ݱ������ݣ��ҳ�60.0gˮ�ܹ��ܽ�66.0g KNO3���¶ȷ�Χ��___________��
��. ����KNO3�л�������NaCl�����ᴿKNO3�����ʵ�鲽��Ϊ________��
��.������ˮ�е��ܽ��Ҳ��һ���ı仯���ɡ���ͼΪ��ͬ�¶��£�ij������ܽ�����¶ȵĹ�ϵ��
ͼ��P1��P2��ʾ��ѹ����P1��P2�Ĺ�ϵ��________��
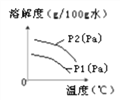
A.P1��P2
B.P1��P2
C.P1��P2
D.��ȷ��
����Ŀ����������װ�ûش����⣺
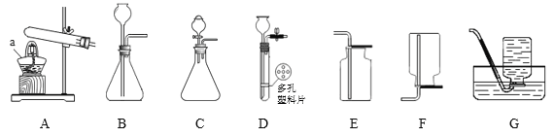
�� д������������ƣ�a ______________��
�� ���������װ��A��������,���Թܿ���ˮ����֣���������______��ѡ���������ѧ�����仯����ġ�
�� �÷�ĩ״̼�����ϡ���ᷴӦ�ƶ�����̼����ʹ��Ӧƽ�Ƚ��еķ���װ����_________��д���÷�Ӧ�Ļ�ѧ����ʽ_______________���ռ�������̼������������Eװ�õ�ԭ����_______________��
�� ̽������������Թ�������ֽ����ʵ�Ӱ�죬ʵ���������±���������Ӱ��ʵ������ؾ���ͬ��
��� | ����������Һ����/�� | ��������/�� | ��������/�� |
ʵ��1 | 100.0 | MnO2 0.2 | t1 |
ʵ��2 | 100.0 | CuO X | t2 |
��.����X����ֵӦ��______��
��.���еĴ�������ָ�����ռ���ͬ�����������ʱ�䣬��t1____��ѡ�>������<����=����t2����MnO2�Ĵ�Ч������CuO���������Բ���__________��Ҳ�ܴﵽʵ��Ŀ�ġ�
��.���ʵ��1����ȫ��Ӧ����������1.6�ˣ������������Һ����������������__________�������ݻ�ѧ����ʽ���㣩