��Ŀ����
�������ƣ�CaO2�����н�ǿ��Ư�ס�ɱ���������ã��Ի�����Σ������һ�ֺ���Ӧ�ü�ֵ�ͷ�չǰ;�Ļ�����Ʒ����ҵ������˫��ˮ�����������Ʊ�CaO2����֪��˫��ˮ�����²��ȶ�����ֽ⣮��ͼ�Ǽ�Ĺ������̣���ش��������⣮

��1����ʯ���м�ˮ�����Ļ�ѧ��Ӧ����ʽ��
��2���������ƽ����м���˫��ˮ�ķ�Ӧ���ڻ��Ϸ�Ӧ����Ӧ����ʽΪ
��3��CaO2��������Ϻ��������еĹ��������䷴Ӧԭ���ǣ�2CaO2+2

��1����ʯ���м�ˮ�����Ļ�ѧ��Ӧ����ʽ��
CaO+H2O=Ca��OH��2
CaO+H2O=Ca��OH��2
���÷�Ӧ������
����
��Ӧ������ȡ������ȡ������ڼ���˫��ˮǰ���������ƽ����辭������
����
����������¡��������¡��������¡�����������2���������ƽ����м���˫��ˮ�ķ�Ӧ���ڻ��Ϸ�Ӧ����Ӧ����ʽΪ
Ca��OH��2+H2O2+6H2O=CaO2?8H2O
Ca��OH��2+H2O2+6H2O=CaO2?8H2O
����õIJ�ƷCaO2?8H2O����Է�������Ϊ216
216
������Ca��O��H��Ԫ��������Ϊ40��160��16
40��160��16
����3��CaO2��������Ϻ��������еĹ��������䷴Ӧԭ���ǣ�2CaO2+2
H2O
H2O
=2Ca��OH��2+O2��������Ҫ�ṩ12.8g����������ҪCaO257.6
57.6
g��Na2O2Ҳ����ˮ��Ӧԭ����CaO2��ͬ����ȴ������Ϊ��Ϻ����Ĺ��������������ԭ��Na2O2��ˮ��Ӧ���ɵ�NaOH��ǿ���ԣ���������Ϻ����
Na2O2��ˮ��Ӧ���ɵ�NaOH��ǿ���ԣ���������Ϻ����
����������1��������ʯ������ˮ�����������������ƣ��ų��������ȣ�д����Ӧ�ķ���ʽ�� ����˫��ˮ�����²��ȶ�����ֽ⣬�������������ƽ��ϵĴ���������
��2����������������˫��ˮ�ķ�Ӧ��д����Ӧ�ķ���ʽ�����ݻ�ѧʽ������Է�����������Ԫ�ص������ȣ�
��3�����������غ㶨�ɷ����հ״������ʣ����ݻ�ѧ����ʽ���������������м�����Ҫ��CaO2����������Na2O2��ˮ��Ӧ������������ʷ�����
��2����������������˫��ˮ�ķ�Ӧ��д����Ӧ�ķ���ʽ�����ݻ�ѧʽ������Է�����������Ԫ�ص������ȣ�
��3�����������غ㶨�ɷ����հ״������ʣ����ݻ�ѧ����ʽ���������������м�����Ҫ��CaO2����������Na2O2��ˮ��Ӧ������������ʷ�����
����⣺��1����ʯ����ˮ�����������������ƣ���Ӧ�ķ���ʽ�ǣ�CaO+H2O=Ca��OH��2���ڸ÷�Ӧ�зų��������ȣ����� ˫��ˮ�����²��ȶ�����ֽ⣮���ԣ�
�������ƽ����辭�����´�����
��2������������˫��ˮ�ķ�Ӧ�ķ���ʽ�ǣ�Ca��OH��2+H2O2+6H2O=CaO2?8H2O��CaO2?8H2O����Է�������Ϊ��40+16��2+��1��2+16����8=216������Ca��O��H��Ԫ��������Ϊ��40����16��10������1��16��=40��160��16��
��3�����������غ㶨�ɿ�֪����Ӧǰ���Ԫ��ԭ�ӵ����༰��Ŀ���䣬�ɷ�Ӧ��֪�հ״�Ӧ�����������H2O��
����Ҫ�ṩ12.8g����������ҪCaO2 ������Ϊx
2CaO2+2H2O=2Ca��OH��2+O2��
144 32
x 12.8g
=
��ã�x=57.6g
�������֪��Na2O����ˮ��Ӧ������NaOH��NaOH��ǿ���ԣ���������Ϻ���森���ԣ�Na2O2������Ϊ��Ϻ����Ĺ�������
�ʴ�Ϊ����1��CaO+H2O=Ca��OH��2 ���ȣ����£���2��Ca��OH��2+H2O2+6H2O=CaO2?8H2O��216��40��160��16����3��H2O��57.6����Na2O2��ˮ��Ӧ���ɵ�NaOH��ǿ���ԣ���������Ϻ���森
�������ƽ����辭�����´�����
��2������������˫��ˮ�ķ�Ӧ�ķ���ʽ�ǣ�Ca��OH��2+H2O2+6H2O=CaO2?8H2O��CaO2?8H2O����Է�������Ϊ��40+16��2+��1��2+16����8=216������Ca��O��H��Ԫ��������Ϊ��40����16��10������1��16��=40��160��16��
��3�����������غ㶨�ɿ�֪����Ӧǰ���Ԫ��ԭ�ӵ����༰��Ŀ���䣬�ɷ�Ӧ��֪�հ״�Ӧ�����������H2O��
����Ҫ�ṩ12.8g����������ҪCaO2 ������Ϊx
2CaO2+2H2O=2Ca��OH��2+O2��
144 32
x 12.8g
| 144 |
| 32 |
| x |
| 12.8g |
�������֪��Na2O����ˮ��Ӧ������NaOH��NaOH��ǿ���ԣ���������Ϻ���森���ԣ�Na2O2������Ϊ��Ϻ����Ĺ�������
�ʴ�Ϊ����1��CaO+H2O=Ca��OH��2 ���ȣ����£���2��Ca��OH��2+H2O2+6H2O=CaO2?8H2O��216��40��160��16����3��H2O��57.6����Na2O2��ˮ��Ӧ���ɵ�NaOH��ǿ���ԣ���������Ϻ���森
������������Ҫ�Ǹ��ݻ�ѧ����ʽ����Ļ������ͣ��ѵ����ڷ�Ӧ��ѧ����ʽ����д��������ѧ������ѧϰ��������

��ϰ��ϵ�д�
 ����������ϵ�д�
����������ϵ�д�
�����Ŀ

 ��2009?Ȫ�����ʼ죩��ĩ��С����ְ־���һ�����ߣ���������ʦ������������һ����ɫ�Ĺ��壬�����ж�ʱ�����������ݣ�����ѯ��֪�����ֹ����׳ơ��㸡�顱����Ҫ�ɷ��ǹ������ƣ�CaO2����
��2009?Ȫ�����ʼ죩��ĩ��С����ְ־���һ�����ߣ���������ʦ������������һ����ɫ�Ĺ��壬�����ж�ʱ�����������ݣ�����ѯ��֪�����ֹ����׳ơ��㸡�顱����Ҫ�ɷ��ǹ������ƣ�CaO2����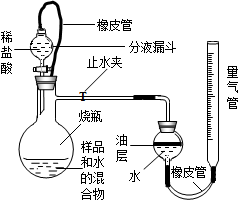 ��2013?�簲����ģ����;��������ʱ�����dz���ˮ�м��������������ƣ�CaO2�����壬Ϊ���ṩ����������������ˮ��Ӧ�����������⣬������ʲô���ʣ�������ȤС���������һ�����н���̽����������룮
��2013?�簲����ģ����;��������ʱ�����dz���ˮ�м��������������ƣ�CaO2�����壬Ϊ���ṩ����������������ˮ��Ӧ�����������⣬������ʲô���ʣ�������ȤС���������һ�����н���̽����������룮