��Ŀ����
ɽ��ʡ�����ذ�ʯ��ɽ��ӵ�зḻ�Ĵ���ʯ�����Դ������̳ɽ�ϵĴ���ʯ��Ʒ������ֻ�������������ʣ�����������һ�ּȲ�����ˮҲ�������ᷴӦ�����µĹ��壩����ʯ����ѧ��ͬѧ����ⶨ����Ʒ��̼��Ƶ���������������ѡȡ��һ�����ʯ��Ʒ�������ô����Ƴ�6g�����ձ��ڣ��ձ�����Ϊ20g����Ȼ�����50gijһ����������������ϡ���ᣬ�ò��������������ٲ�������Ϊֹ����Ӧ����ʱ�䣨t�����ձ�������ʢ������������m���Ĺ�ϵ��ͼ��ʾ��������������⣺
��1��������ʯ��Ʒ�ô�������ҪĿ���ǣ�______��
��2��ʵ�����ʱ�������Ϲ��ų�������̼������Ϊ______g��
��3�������ʯ��ʯ��Ʒ��̼��Ƶ�����������������С�����һλС����
��1��������ʯ��Ʒ�ô�������ҪĿ���ǣ�______��
��2��ʵ�����ʱ�������Ϲ��ų�������̼������Ϊ______g��
��3�������ʯ��ʯ��Ʒ��̼��Ƶ�����������������С�����һλС����

��1��Ϊ������Ʒ��̼��Ʋ�����ɷ�Ӧ���ɰ���Ʒ���飬������Ʒ������ĽӴ�������˾ٻ����Լӿ췴Ӧ�����ʣ�
�ʴ�Ϊ������Ӵ�������ӿ췴Ӧ���ʣ�
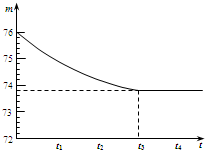
��2��ͼ����������ÿ��С�̶ȱ�ʾ0.2g��������ȫ�ų������ʣ������������Ϊ73.8g��
���������غ㶨�ɣ���˷ų����������̼������=76g-73.8g=2.2g
�ʴ�Ϊ��2.2g��
��3����ʯ��ʯ��Ʒ��̼��Ƶ�����Ϊx��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 2.2g
=
��֮�ã�x=5g
ʯ��ʯ��Ʒ��̼��Ƶ���������=
��100%=83.3%��
�𣺸�ʯ��ʯ��Ʒ��̼��Ƶ�����������83.3%��
�ʴ�Ϊ������Ӵ�������ӿ췴Ӧ���ʣ�
��2��ͼ����������ÿ��С�̶ȱ�ʾ0.2g��������ȫ�ų������ʣ������������Ϊ73.8g��
���������غ㶨�ɣ���˷ų����������̼������=76g-73.8g=2.2g
�ʴ�Ϊ��2.2g��
��3����ʯ��ʯ��Ʒ��̼��Ƶ�����Ϊx��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 2.2g
| 100 |
| 44 |
| x |
| 2.2g |
��֮�ã�x=5g
ʯ��ʯ��Ʒ��̼��Ƶ���������=
| 5g |
| 6g |
�𣺸�ʯ��ʯ��Ʒ��̼��Ƶ�����������83.3%��

��ϰ��ϵ�д�
�����Ŀ