��Ŀ����
�������ƾ���(CaO2��nH2O)������Ϊ��ɫ���������ᣬ������ˮ���Ҵ�����һ���º͵������������������;������������ȡ�
l.�������ƾ���(CaO2��nH2O)���Ʊ�
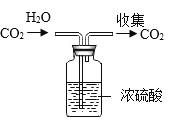
ʵ�����Ʊ��������ƾ��巴Ӧ����ʽΪCaCl2��H2O2��2NH3��nH2O=CaO2?nH2O+2NH4Cl��ʵ��װ����ͼ��
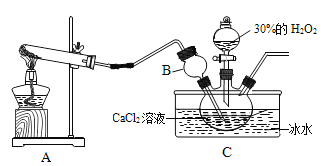
��1��װ��A�����ɵ�����Ϊ______��
��2��װ��B��������________��
��3��װ��C���ñ�ˮԡ�����¶���0�����ң����ܵ�ԭ����Ҫ�У�
�ٸ÷�Ӧ�Ƿ��ȷ�Ӧ���¶ȵ����������CaO2?nH2O���ʣ�
��____��
��4����Ӧ���������ˡ�ϴ�ӡ����º�ɿɻ��CaO2?nH2O�����龧����ϴ�Ӹɾ��ķ���Ϊ______��
��.�������ƾ���(CaO2?nH2O)��n�IJⶨ
���ϣ�CaO2?nH2O������120����ȫʧȥ�ᾧˮ������������350�棬ʣ��������ȷֽ�ų�O2��
��5��ij��ȤС���ȡ2.16g CaO2?nH2O��Ʒ�����ȷ����Ƕ�������ȷֽ�ʵ�飬���ʣ�������������¶ȱ仯������ͼ��ʾ
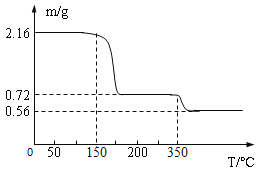
��CaO2?nH2O��n��ֵΪ____��(д���������)
��350�淢����Ӧ�Ļ�ѧ����ʽΪ_________��
������������Ʒ���л�������(���ʲ��μӷ�Ӧ)���ᵼ�²�õ�n��ֵ___(�ƫ��ƫС�����䡱)��
��.�������ƾ���(CaO2?nH2O)�������о�
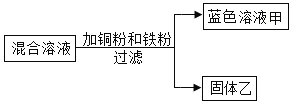
��6������ȤС������ȡ����CaO2?nH2O��Ʒ���Թ��У�����������ϡ����ʹ��Ʒ��ȫ��Ӧ��Ȼ�����������Na2CO3��Һ������_____����˵��CaO2?nH2O��ϡ���ᷴӦ������CaCl2��



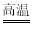 2Fe+Al2O3��X�Ļ�ѧʽΪ______���˷�Ӧ�Ļ�����Ӧ������_____��
2Fe+Al2O3��X�Ļ�ѧʽΪ______���˷�Ӧ�Ļ�����Ӧ������_____��