��Ŀ����
Ϊ�ⶨijʯ��ʯ��Ʒ��CaCO3������������ȡʯ��ʯ��Ʒ������ϡ�������ձ��з�Ӧ������ʯ��ʯ��Ʒ�����ʲ���ϡ���ᷴӦ��Ҳ������ˮ���й�ʵ���������±���
| ��Ӧǰ | ��Ӧ�� | ||
| ʵ�� ���� | �ձ���ϡ��������� | ʯ��ʯ��Ʒ������ | �ձ���ʣ��������� |
| 150 �� | 12�� | 157.6�� | |
��2�����ʯ��ʯ�к�CaCO3������������
�⣺��1�����������غ㶨�ɣ�������̼������=150g+12g-157.6g=4.4g��
�ʴ�Ϊ��4.4�ˣ�
��2����̼��Ƶ�����Ϊx
CaCO3+2HCl=CaCl2+CO2��+H2O
100 44
x 4.4g
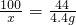
x=10g
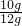 ��100%��83.3% �𣺸�ʯ��ʯ�к�CaCO3������������83.3%��
��100%��83.3% �𣺸�ʯ��ʯ�к�CaCO3������������83.3%��
�ʴ�Ϊ��83.3%��
�������ڱ����У������������ݿ�֪�����ٵ������Ƕ�����̼�����������ݶ�����̼�������������̼��Ƶ�������Ȼ������̼��Ƶ��������������ݻ�ѧ����ʽ����IJ����У�һ�衢��д�����ҡ����С�������飮ע�������У���δ֪��������λ�����������Ҫ����λ�����Ҫ����λ��
�����������㿼���˸��ݻ�ѧ����ʽ�ļ��㣬���ڱ����ͼ����⣮Ҫ������á�������ֵ�����塱�����������غ㶨���������������Ȼ�������������ʵ�������Ҫ�������������ݣ�����ʱҪע�⣺��ѧ����ʽҪд��ȷ��ʼ�ղ�Ҫ���������غ㶨�ɣ���������Ҫ�����ڼ������У�
�ʴ�Ϊ��4.4�ˣ�
��2����̼��Ƶ�����Ϊx
CaCO3+2HCl=CaCl2+CO2��+H2O
100 44
x 4.4g
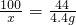
x=10g
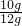 ��100%��83.3% �𣺸�ʯ��ʯ�к�CaCO3������������83.3%��
��100%��83.3% �𣺸�ʯ��ʯ�к�CaCO3������������83.3%���ʴ�Ϊ��83.3%��
�������ڱ����У������������ݿ�֪�����ٵ������Ƕ�����̼�����������ݶ�����̼�������������̼��Ƶ�������Ȼ������̼��Ƶ��������������ݻ�ѧ����ʽ����IJ����У�һ�衢��д�����ҡ����С�������飮ע�������У���δ֪��������λ�����������Ҫ����λ�����Ҫ����λ��
�����������㿼���˸��ݻ�ѧ����ʽ�ļ��㣬���ڱ����ͼ����⣮Ҫ������á�������ֵ�����塱�����������غ㶨���������������Ȼ�������������ʵ�������Ҫ�������������ݣ�����ʱҪע�⣺��ѧ����ʽҪд��ȷ��ʼ�ղ�Ҫ���������غ㶨�ɣ���������Ҫ�����ڼ������У�

��ϰ��ϵ�д�
 ����ѧУ�ֲ����ܲ�ϵ�д�
����ѧУ�ֲ����ܲ�ϵ�д� �ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�����Ŀ
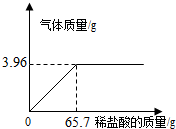 ����С��Ϊ�ⶨijʯ��ʯ��Ʒ��CaCO3�ĺ�����ȡl0gʯ��ʯ��Ʒ�����ձ��У�Ȼ��������ע��һ����ij����������ϡ���ᣬʹ֮����Ʒ��ַ�Ӧ�����ʲ����뷴Ӧ�����淴Ӧ���У�ע��ϡ����������뷴Ӧ�õ��������������ͼ��ʾ��ϵ����������м������ݣ�
����С��Ϊ�ⶨijʯ��ʯ��Ʒ��CaCO3�ĺ�����ȡl0gʯ��ʯ��Ʒ�����ձ��У�Ȼ��������ע��һ����ij����������ϡ���ᣬʹ֮����Ʒ��ַ�Ӧ�����ʲ����뷴Ӧ�����淴Ӧ���У�ע��ϡ����������뷴Ӧ�õ��������������ͼ��ʾ��ϵ����������м������ݣ�