��Ŀ����
С��ͬѧΪ�˲ⶨ�������۵�ijƷ�ƴ������Ϊ�Ȼ��ƣ���̼���Ƶ���������������������ʵ�飺���������ձ��зֱ����11.0g��Ʒ��������ˮ�����Һ�����������ձ��зֱ����һ��������10.0%�Ȼ�����Һ���۽���ַ�Ӧ�����ɵij������ˡ�ϴ�ӡ�����������õ��İ�ɫ���塣
ʵ���������ݼ��±�����������������йؼ��㣺
| �ձ��� | �ձ��� | �ձ��� | |
| ����10.0%�Ȼ�����Һ��������g�� | 55.5 | 120.0 | 150.0 |
| ��ɫ�����������g�� | 5.0 | 10.0 | 10.0 |
��1����Ʒ��̼���Ƶ����������Ƕ��٣�(�����ȷ��0.1%)
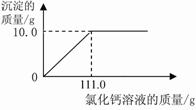
��2������ͼ�л��Ƴ���ɫ����������Ȼ�����Һ��������ϵͼ��ͼ��Ҫע����ɫ�������Ȼ�����Һ�������������
��ʵ�����ݿ�֪��11.0g��Ʒ�е�̼�������Ȼ�����Һ��ַ�Ӧ�������10.0g̼��ơ�
(1)�⣺��Ҫ����10.0g̼�����Ҫ̼���Ƶ�����ΪX
Na2CO3 + CaCl2 = CaCO3��+ 2NaCl
106 100
X 10.0g
106:100 = X��10.0g
���X=10.6g
��Ʒ��̼���Ƶ���������Ϊ�� 10.6g/11.0g��100%=96.4% ��1�֣�
 (2)
(2)

С��ͬѧΪ�˲ⶨ�������۵�ijƷ�ƴ������Ϊ�Ȼ��ƣ���̼���Ƶ���������������������ʵ�飺���������ձ��зֱ����11.0g��Ʒ��������ˮ�����Һ�����������ձ��зֱ����һ��������10.0%�Ȼ�����Һ���۽���ַ�Ӧ�����ɵij������ˡ�ϴ�ӡ�����������õ��İ�ɫ���塣
ʵ���������ݼ��±�����������������йؼ��㣺
| �ձ��� | �ձ��� | �ձ��� | |
| ����10.0%�Ȼ�����Һ��������g�� | 55.5 | 120.0 | 150.0 |
| ��ɫ�����������g�� | 5.0 | 10.0 | 10.0 |
��1����Ʒ��̼���Ƶ����������Ƕ��٣�(�����ȷ��0.1%)
��2������ͼ�л��Ƴ���ɫ����������Ȼ�����Һ��������ϵͼ��ͼ��Ҫע����ɫ�������Ȼ�����Һ�������������


С��ͬѧΪ�˲ⶨ�������۵�ijƷ�ƴ������Ϊ�Ȼ��ƣ���̼���Ƶ���������������������ʵ�飺���������ձ��зֱ����11.0g��Ʒ��������ˮ�����Һ�����������ձ��зֱ����һ��������10.0%�Ȼ�����Һ���۽���ַ�Ӧ�����ɵij������ˡ�ϴ�ӡ�����������õ��İ�ɫ���壮
ʵ���������ݼ��±�����������������йؼ��㣺
| �ձ��� | �ձ��� | �ձ��� | |
| ����10.0%�Ȼ�����Һ��������g�� | 55.5 | 120.0 | 150.0 |
| ��ɫ�����������g�� | 5.0 | 10.0 | 10.0 |
��1����Ʒ��̼���Ƶ����������Ƕ��٣��������ȷ��0.1%��
��2������ͼ�л��Ƴ���ɫ����������Ȼ�����Һ��������ϵͼ��ͼ��Ҫע��
��ɫ�������Ȼ�����Һ�������������

| |
 ��2012?̩����С��ͬѧΪ�˲ⶨ�������۵�ijƷ�ƴ������Ϊ�Ȼ��ƣ���̼���Ƶ���������������������ʵ�飺���������ձ��зֱ����11.0g��Ʒ��������ˮ�����Һ�����������ձ��зֱ����һ��������10.0%�Ȼ�����Һ���۽���ַ�Ӧ�����ɵij������ˡ�ϴ�ӡ�����������õ��İ�ɫ���壮
��2012?̩����С��ͬѧΪ�˲ⶨ�������۵�ijƷ�ƴ������Ϊ�Ȼ��ƣ���̼���Ƶ���������������������ʵ�飺���������ձ��зֱ����11.0g��Ʒ��������ˮ�����Һ�����������ձ��зֱ����һ��������10.0%�Ȼ�����Һ���۽���ַ�Ӧ�����ɵij������ˡ�ϴ�ӡ�����������õ��İ�ɫ���壮
