��Ŀ����

��2������ͼ�л��Ƴ���ɫ����������Ȼ�����Һ��������ϵͼ��ͼ��Ҫע����ɫ�������Ȼ�����Һ�������������

��1����Ҫ����10.0g̼�����Ҫ̼���Ƶ�����ΪX
Na2CO3+CaCl2=CaCO3+2NaCl
106 100
x 10.0g 
��ã�x=10.6g
��Ʒ��̼���Ƶ���������Ϊ��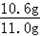 ��100%=96.4%��
��100%=96.4%��
����Ʒ��̼���Ƶ�����������96.4%��
��2������ͼ����Ϣ����֪���Ƚ��ձ�����ձ����е����ݿ���֪����55.5g�Ȼ�����Һ���Ժ�̼���Ʒ�Ӧ����5.0g��������ô����10.0g�����������Ȼ�����Һ������Ϊ111.0g�����Ծݴ���ͼΪ��
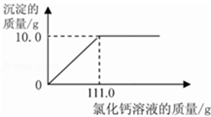

С��ͬѧΪ�˲ⶨ�������۵�ijƷ�ƴ������Ϊ�Ȼ��ƣ���̼���Ƶ���������������������ʵ�飺���������ձ��зֱ����11.0g��Ʒ��������ˮ�����Һ�����������ձ��зֱ����һ��������10.0%�Ȼ�����Һ���۽���ַ�Ӧ�����ɵij������ˡ�ϴ�ӡ�����������õ��İ�ɫ���塣
ʵ���������ݼ��±�����������������йؼ��㣺
| �ձ��� | �ձ��� | �ձ��� | |
| ����10.0%�Ȼ�����Һ��������g�� | 55.5 | 120.0 | 150.0 |
| ��ɫ�����������g�� | 5.0 | 10.0 | 10.0 |
��1����Ʒ��̼���Ƶ����������Ƕ��٣�(�����ȷ��0.1%)
��2������ͼ�л��Ƴ���ɫ����������Ȼ�����Һ��������ϵͼ��ͼ��Ҫע����ɫ�������Ȼ�����Һ�������������


С��ͬѧΪ�˲ⶨ�������۵�ijƷ�ƴ������Ϊ�Ȼ��ƣ���̼���Ƶ���������������������ʵ�飺���������ձ��зֱ����11.0g��Ʒ��������ˮ�����Һ�����������ձ��зֱ����һ��������10.0%�Ȼ�����Һ���۽���ַ�Ӧ�����ɵij������ˡ�ϴ�ӡ�����������õ��İ�ɫ���壮
ʵ���������ݼ��±�����������������йؼ��㣺
| �ձ��� | �ձ��� | �ձ��� | |
| ����10.0%�Ȼ�����Һ��������g�� | 55.5 | 120.0 | 150.0 |
| ��ɫ�����������g�� | 5.0 | 10.0 | 10.0 |
��1����Ʒ��̼���Ƶ����������Ƕ��٣��������ȷ��0.1%��
��2������ͼ�л��Ƴ���ɫ����������Ȼ�����Һ��������ϵͼ��ͼ��Ҫע��
��ɫ�������Ȼ�����Һ�������������

| |
С��ͬѧΪ�˲ⶨ�������۵�ijƷ�ƴ������Ϊ�Ȼ��ƣ���̼���Ƶ���������������������ʵ�飺���������ձ��зֱ����11.0g��Ʒ��������ˮ�����Һ�����������ձ��зֱ����һ��������10.0%�Ȼ�����Һ���۽���ַ�Ӧ�����ɵij������ˡ�ϴ�ӡ�����������õ��İ�ɫ���塣
ʵ���������ݼ��±�����������������йؼ��㣺
| �ձ��� | �ձ��� | �ձ��� | |
| ����10.0%�Ȼ�����Һ��������g�� | 55.5 | 120.0 | 150.0 |
| ��ɫ�����������g�� | 5.0 | 10.0 | 10.0 |
��1����Ʒ��̼���Ƶ����������Ƕ��٣�(�����ȷ��0.1%)
��2������ͼ�л��Ƴ���ɫ����������Ȼ�����Һ��������ϵͼ��ͼ��Ҫע����ɫ�������Ȼ�����Һ�������������
 ��2012?̩����С��ͬѧΪ�˲ⶨ�������۵�ijƷ�ƴ������Ϊ�Ȼ��ƣ���̼���Ƶ���������������������ʵ�飺���������ձ��зֱ����11.0g��Ʒ��������ˮ�����Һ�����������ձ��зֱ����һ��������10.0%�Ȼ�����Һ���۽���ַ�Ӧ�����ɵij������ˡ�ϴ�ӡ�����������õ��İ�ɫ���壮
��2012?̩����С��ͬѧΪ�˲ⶨ�������۵�ijƷ�ƴ������Ϊ�Ȼ��ƣ���̼���Ƶ���������������������ʵ�飺���������ձ��зֱ����11.0g��Ʒ��������ˮ�����Һ�����������ձ��зֱ����һ��������10.0%�Ȼ�����Һ���۽���ַ�Ӧ�����ɵij������ˡ�ϴ�ӡ�����������õ��İ�ɫ���壮