��Ŀ����
����Ŀ����ѧ�ͻ���������ʳƷӪ��������Ӧ�õ�������ء�
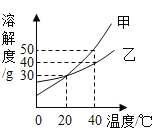
��1����������̿+����Ĥ+����������Ϲ��ջ��ֱ��ˮ�����л���̿��������______��
��2������������ʳ�õ�������ʳ���У����������ʵ���______(�����)��

��3��ʹ�õ綯�۹����Ч������CO2��SO2��CO��������ŷţ���Щ����������������ЧӦ����______��������������______��
��4�����������У�����������̼�������������______��
A ���������������ᳫʹ�ý��ܼ���
B ����ѡ�ù�����ͨ���ٿ���
C ���ദ����������
D ������ˮ��24Сʱ����
���𰸡����� C CO2 SO2 D
��������
(1)��ˮ�����У�����̿���������ã�����ˮ�е�ɫ�غ���ζ������д��������
(2)���Ѹ���ά���أ����������֬�������и��������ʣ�����д��C��
(3)���������У�����������ЧӦ����CO2������д��CO2��
������������SO2�������SO2��
(4)����̼�������������ָҪ�������������������õ���������Ҫ�Ǵӽڵ硢�����ͻ���������������������̼������������д��D��

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�����Ŀ��ijУ��ѧ��ȤС��ι��Ƽ���������Ϣ���������������о���
���������ϣ�
����ˮ����ͭ�ǰ�ɫ���壬��ˮ������
������ԭ�ϴ����к����������������ʣ�![]() ��
��![]() �������������ʣ��������������Ƚ����ξ��ơ�
�������������ʣ��������������Ƚ����ξ��ơ�
������ԭ����![]() ������þ���A����ʹ�������ȣ��ɷֽ��Ƶô�����ֳ����������
������þ���A����ʹ�������ȣ��ɷֽ��Ƶô�����ֳ����������
���Ȼ�立ֽ�Ļ�ѧ����ʽ�ǣ�![]() ��
��
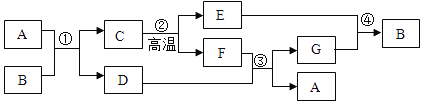
�ݲ�������������ͼ��ʾ��
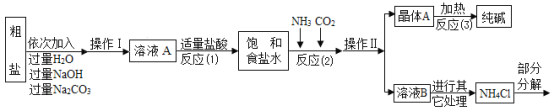
���������ۣ�
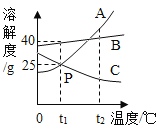
��1����д������NaOH��Һ��������Ӧ�Ļ�ѧ����ʽ________��
�ڷ�Ӧ��1���м������������Ŀ����________��
��2���������������п�ѭ��ʹ�õ�������________������ĸ����
A![]() B NaOH C HCl D
B NaOH C HCl D![]()
�����̽��һ��
��3���پ���A���ȷֽ�Ļ�ѧ����ʽΪ________��
�����ʵ����鴿����Ʒ���Ƿ���о���A��������±���װ�ô���ͼ��ѡ��
ѡ���װ�� | ʵ������ | ʵ����� |
________������ĸ�� | ________ | ��Ʒ��������A |
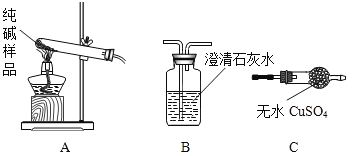
�����̽������
��4��ȡ������Ʒ��ˮ�ܽ⣬�����Һ�м������ϡ![]() ���ٵμ�
���ٵμ�![]() ��Һ���а�ɫ���������������Ļ�ѧ����ʽΪ________���ɴ�ȷ��������Ʒ��������NaCl��
��Һ���а�ɫ���������������Ļ�ѧ����ʽΪ________���ɴ�ȷ��������Ʒ��������NaCl��
�����̽������
��5��ͬѧ��Ϊ�˲ⶨ�ô�����Ʒ�Ĵ��ȣ����������ʵ�飺
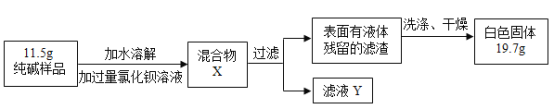
���Ȼ�����Һ������Ŀ����________���ж��Ȼ����ѹ����ķ�����________��
���ж������Ƿ�ϴ���ķ����ǣ�ȡ���һ��ϴ��Һ�������Թ��У��μ�________������������������ϴ����
�۸���ʵ�����ݣ�������Ʒ��̼���Ƶ���������Ϊ________���г�������̣�����һλС������