题目内容
(9分)化学实验是学习化学的基础,请根据下列装置图回答问题。
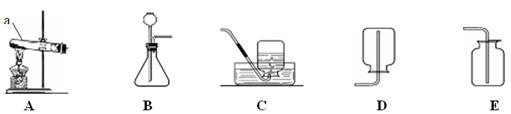
 (1)A图中制取CO2的化学方程式为 ,浓硫酸的作用是 。
(1)A图中制取CO2的化学方程式为 ,浓硫酸的作用是 。
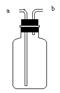
(2)将接口a和b连接,可观察到,仅有下面玻璃管内湿润的石蕊试纸变红,上面玻璃管内无明显现象产生。据此说明;①二氧化碳气体密度比空气密度 ;②二氧化碳与水反应生成显 性物质。
(3)已知:①CaCO3+CO2+H2O= Ca(HCO3)2;②Ca(HCO3)2易溶于水,能与澄清石灰水或碳酸钠溶液反应都生成碳酸钙沉淀,也能与盐酸反应放出二氧化碳气体。将接口a和c连接,通入一定量的二氧化碳气体后,将试管静置,是固液分离,则上层溶液中可能含有的溶质是Ca(OH)2或 。请你设计实验方案加以验证:
 (1)A图中制取CO2的化学方程式为 ,浓硫酸的作用是 。
(1)A图中制取CO2的化学方程式为 ,浓硫酸的作用是 。(2)将接口a和b连接,可观察到,仅有下面玻璃管内湿润的石蕊试纸变红,上面玻璃管内无明显现象产生。据此说明;①二氧化碳气体密度比空气密度 ;②二氧化碳与水反应生成显 性物质。
(3)已知:①CaCO3+CO2+H2O= Ca(HCO3)2;②Ca(HCO3)2易溶于水,能与澄清石灰水或碳酸钠溶液反应都生成碳酸钙沉淀,也能与盐酸反应放出二氧化碳气体。将接口a和c连接,通入一定量的二氧化碳气体后,将试管静置,是固液分离,则上层溶液中可能含有的溶质是Ca(OH)2或 。请你设计实验方案加以验证:
| 实验操作 | 实验现象 | 结论 |
| 取少量上层溶液于试管中,然后滴加 | | 溶质含有 |
(1)CaCO3 + 2HCl ="=" CaCl2 + 2H2O + CO2↑ 干燥二氧化碳气体 (2)①大 ②酸(3)Ca(HCO3)2 盐酸 放出气体 Ca(HCO3)2(合理答案即可)
(1)石灰石的主要成分是碳酸钙,碳酸钙和盐酸反应生成氯化钙和水和二氧化碳.二氧化碳与浓硫酸不反应,因此可以用浓硫酸进行干燥.故答案为:CaCO3+2HCl═CaCl2+H2O+CO2↑;干燥二氧化碳气体.
(2)将接口a和b连接,可观察到,仅有下面玻璃管内湿润的石蕊试纸变红,上面玻璃管内无明显现象产生.说明:二氧化碳气体密度比空气密度大,二氧化碳与水反应生成物显酸性.故答案为:①大;②酸.
(3)CaCO3+CO2+H2O=Ca(HCO3)2,Ca(HCO3)2易溶于水,能与盐酸反应放出二氧化碳气体,因此Ca(OH)2和Ca(HCO3)2进行检验时,一般用稀盐酸,有气泡冒出就有Ca(HCO3)2,没有气泡冒出,就是氢氧化钙.故答案为: Ca(HCO3)2;盐酸;放出气体;Ca(HCO3)2(合理答案即可)
(2)将接口a和b连接,可观察到,仅有下面玻璃管内湿润的石蕊试纸变红,上面玻璃管内无明显现象产生.说明:二氧化碳气体密度比空气密度大,二氧化碳与水反应生成物显酸性.故答案为:①大;②酸.
(3)CaCO3+CO2+H2O=Ca(HCO3)2,Ca(HCO3)2易溶于水,能与盐酸反应放出二氧化碳气体,因此Ca(OH)2和Ca(HCO3)2进行检验时,一般用稀盐酸,有气泡冒出就有Ca(HCO3)2,没有气泡冒出,就是氢氧化钙.故答案为: Ca(HCO3)2;盐酸;放出气体;Ca(HCO3)2(合理答案即可)

练习册系列答案
相关题目
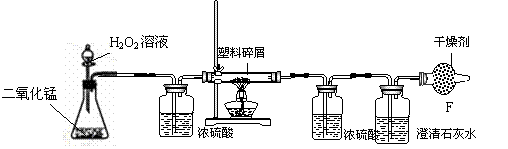

 根据上述实验操作过程,。请找出乙同学的操作错误的是(填序号)__________、 。
根据上述实验操作过程,。请找出乙同学的操作错误的是(填序号)__________、 。