��Ŀ����
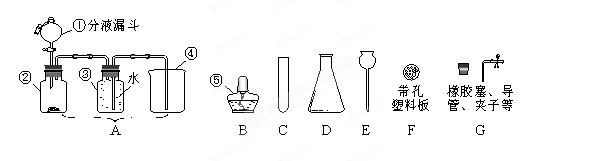
��14�֣���1��ʵ�����г�������غͶ������̼�����������п����ϡ���ᷴӦ��������ʯ��ʯ��ϡ�����ƶ�����̼���塣
A B C D E
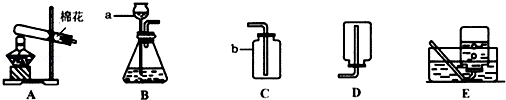
��1��д��������������Ƣ� ��
��������ҩƷѡ���ʵ������巢�����ռ�װ�������±���������ĸ��
��2��д��ʵ������ʯ��ʯ��ϡ�����ƶ�����̼����Ļ�ѧ����ʽ��
��3�����ڴ���ʹ��һ�������Ϸ������ɵġ���ɫ��Ⱦ�����ѳ�Ϊһ�����ص�������⡣ij��ѧ�о�С���ͬѧ����ij�����ϴ�����ɽ��з���̽����������ʾ������ֻ��C��H����Ԫ�أ��������������ͼ��ʾ��ʵ��װ�ã�ʹ�����������ڴ�����ȼ�գ��۲�ʵ���������й����ݣ�����Ԫ�غ�����
A B C D E
������B�������� ��
������E������� ��
��������C�IJ������з����������������ΪW g�������������ȼ�պ�������D����a g����W g�����������к���Ԫ�ص�����Ϊ g����������Ϊ������ʽ��
����װ����û����������B����ʹ��������������Ԫ�ص�����������
���ƫС������ƫ������Ӱ�족��֮һ����

A B C D E
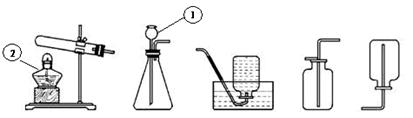
��1��д��������������Ƣ� ��
��������ҩƷѡ���ʵ������巢�����ռ�װ�������±���������ĸ��
| ��ȡ������ | ����װ�� | �ռ�װ�� |
| O2 | | |
| H2 | | |
| CO2 | | |
��2��д��ʵ������ʯ��ʯ��ϡ�����ƶ�����̼����Ļ�ѧ����ʽ��
��3�����ڴ���ʹ��һ�������Ϸ������ɵġ���ɫ��Ⱦ�����ѳ�Ϊһ�����ص�������⡣ij��ѧ�о�С���ͬѧ����ij�����ϴ�����ɽ��з���̽����������ʾ������ֻ��C��H����Ԫ�أ��������������ͼ��ʾ��ʵ��װ�ã�ʹ�����������ڴ�����ȼ�գ��۲�ʵ���������й����ݣ�����Ԫ�غ�����

A B C D E
������B�������� ��
������E������� ��
��������C�IJ������з����������������ΪW g�������������ȼ�պ�������D����a g����W g�����������к���Ԫ�ص�����Ϊ g����������Ϊ������ʽ��
����װ����û����������B����ʹ��������������Ԫ�ص�����������
���ƫС������ƫ������Ӱ�족��֮һ����
��1���ٳ���©�� �ھƾ���
��2��CaCO3 + 2HCl="===" CaCl2 + H2O+ CO2��
��3���ٸ����A����װ���г�������������������CO2���ɣ�Eƿ����ֻ��� �� a/9 ��ƫ��
| ��ȡ������ | ����װ�� | �ռ�װ�� |
| O2 | A | C��D |
| H2 | B | E |
| CO2 | B | D |
��2��CaCO3 + 2HCl="===" CaCl2 + H2O+ CO2��
��3���ٸ����A����װ���г�������������������CO2���ɣ�Eƿ����ֻ��� �� a/9 ��ƫ��
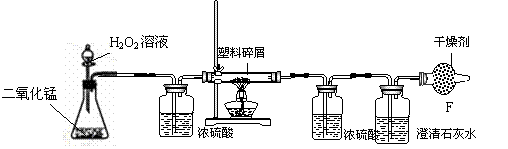
��1������©�����ƾ���Ϊ���л�ѧ������������Ϊ����©������Ϊ�ƾ���
��2��ʵ��������ʯ��ʯ��ϡ������ȡ������̼����ķ�Ӧԭ��ΪCaCO3 + 2HCl="===" CaCl2 + H2O+ CO2��
��3����װ��B��װ����Ũ���ᣬŨ�������ڳ�ˮ������
��װ��E��װ���dz����ʯ��ˮ�����������������̼�ģ�������̼��ʹ�����ʯ��ˮ����ǣ�
�������������ȼ�պ�Ũ���������ˮ�ԣ���D���ص���������W g����������ȼ�����ɵ�ˮ��������װ��D����a g���������غ㶨�ɣ�ˮ����Ԫ�ؼ�������Ʒ�е���Ԫ�أ�����W g�����������к���Ԫ�ص�����Ϊ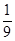 ag��
ag��
��������װ����û����������B����ʹ�����е�ˮ�������װ��D���Ӷ�ʹʵ���õ�ˮ����������������Ʒȼ�����ɵ�ˮ���ʽ�ʹ��������������Ԫ�ص�����������ƫ��
��2��ʵ��������ʯ��ʯ��ϡ������ȡ������̼����ķ�Ӧԭ��ΪCaCO3 + 2HCl="===" CaCl2 + H2O+ CO2��
��3����װ��B��װ����Ũ���ᣬŨ�������ڳ�ˮ������
��װ��E��װ���dz����ʯ��ˮ�����������������̼�ģ�������̼��ʹ�����ʯ��ˮ����ǣ�
�������������ȼ�պ�Ũ���������ˮ�ԣ���D���ص���������W g����������ȼ�����ɵ�ˮ��������װ��D����a g���������غ㶨�ɣ�ˮ����Ԫ�ؼ�������Ʒ�е���Ԫ�أ�����W g�����������к���Ԫ�ص�����Ϊ
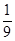 ag��
ag����������װ����û����������B����ʹ�����е�ˮ�������װ��D���Ӷ�ʹʵ���õ�ˮ����������������Ʒȼ�����ɵ�ˮ���ʽ�ʹ��������������Ԫ�ص�����������ƫ��

��ϰ��ϵ�д�
�����Ŀ
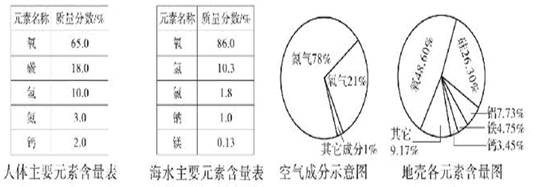

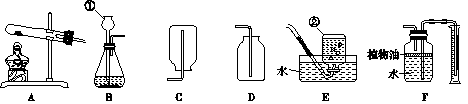


 ��1��Aͼ����ȡCO2�Ļ�ѧ����ʽΪ ��Ũ����������� ��
��1��Aͼ����ȡCO2�Ļ�ѧ����ʽΪ ��Ũ����������� ��