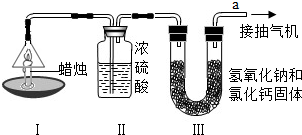
��Ŀ����
Ϊ�ⶨ������̼���������Ʒ�Ӧ�IJ��ij��ѧѧϰС���ͬѧ��һ������NaOH��Һ��ͨ��һ������CO2��ַ�Ӧ���������ᾧ���õ���ɫ���壬�Ըð�ɫ����ijɷ֡�
[���Ͽ�]��������̼����ʱ��̼����Ҳ���������̼��ӦNa2CO3+CO2+H2O=2NaHCO3
Na2CO3+HCl=NaHCO3+NaCl�� NaHCO3+HCl=NaCl+H2O+CO2����
NaHCO3+NaOH=Na2CO3+H2O�� 2NaHCO3 ���� Na2CO3+CO2��+H2O��
Na2CO3��NaOH�����ȶ��Զ��Ϻá�
[���������]��С���ͬѧ�����˲²⣺
(1)��ͬѧ��Ϊ�ð�ɫ���������NaOH��Na2CO3�Ļ�������Ϊ�ù��廹������Na2CO3��____________��____________��
[ʵ�����]
(2)
| ʵ����� | ʵ������ | ���� |
| (1)ȡһ����������Ʒ������ | ������������ | һ���� һ���� ������ |
| (2)��ȡ��Ʒ�������еμ�ϡ���� | ��������� |
��1��Na2CO3��NaHCO3��1�֣� NaHCO3��1�֣� ��2��һ����NaHCO3��1�֣� һ��û��NaOH��1�֣� ������Na2CO3

��ϰ��ϵ�д�
 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д� �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д�
�����Ŀ

 ��1��ʱ����Щ������ϲ��Ⱦ����Ⱦ��ʱһ��Ҫ�õ�һ����ɫ��--�Ա��������仯ѧʽΪC6H8N2������һ���ж��Ļ�ѧҩƷ�����Ⱦ���ߵ���������˺���
��1��ʱ����Щ������ϲ��Ⱦ����Ⱦ��ʱһ��Ҫ�õ�һ����ɫ��--�Ա��������仯ѧʽΪC6H8N2������һ���ж��Ļ�ѧҩƷ�����Ⱦ���ߵ���������˺���
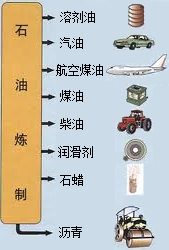 �й�ʯ����Ȼ�����Ź�˾�����������ڲ�����̲���������ִ�����ģ��ʮ�ڶֵĴ�����--�����ϱ���������ǿ�ҹ���Դ��ȫ��Ӧ�ı�������������Ҫ���壮ʯ������Ҫ����̼��������Ԫ�أ�����ʯ���и��ɷֵķе㲻ͬ�������Ƿ��루��Ϊ�����ɵõ���ͬ�IJ�Ʒ��ʹʯ�͵õ��ۺ����ã���ͼ����
�й�ʯ����Ȼ�����Ź�˾�����������ڲ�����̲���������ִ�����ģ��ʮ�ڶֵĴ�����--�����ϱ���������ǿ�ҹ���Դ��ȫ��Ӧ�ı�������������Ҫ���壮ʯ������Ҫ����̼��������Ԫ�أ�����ʯ���и��ɷֵķе㲻ͬ�������Ƿ��루��Ϊ�����ɵõ���ͬ�IJ�Ʒ��ʹʯ�͵õ��ۺ����ã���ͼ����