��Ŀ����
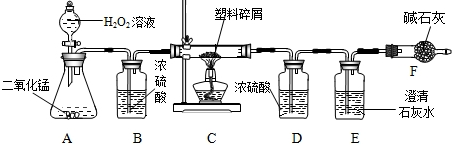
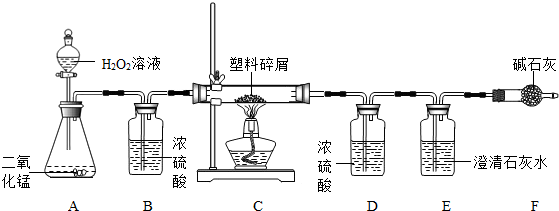
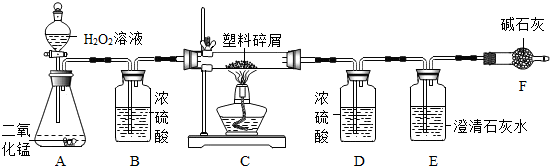
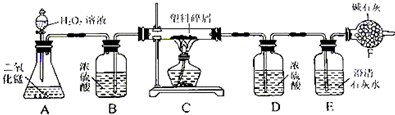
���ڴ���ʹ��һ�������Ϸ��������ɵġ���ɫ��Ⱦ���ѳ�Ϊһ�����ص�������⡣ij��ѧ�о�С���ͬѧ��ij�����ϴ�����ɽ��з����о�( ������ʾ������ֻ��C ��H ����Ԫ��) �������������ͼ��ʾ��ʵ��װ�ã�ʹ�����������ڴ�������ȫȼ�գ��۲�ʵ���������й����ݡ�����Ԫ�غ���������ʯ�ҿ�����ˮ�Ͷ�����̼��
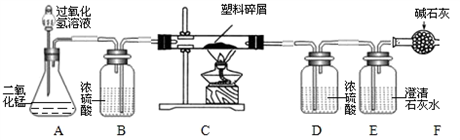
(1) ʵ��װ������һ�����Դ�����д���������� _________ .
(2) װ��A �з�Ӧ�Ļ�ѧ����ʽΪ ___ .
(3) װ��E �е������� _______________��װ��F �������� ____________________________.
(4) ��װ��C �IJ������з����������������Ϊ5.9g �������������ȼ�պ�װ��D ����7.2g ��������������к���Ԫ�ص�����Ϊ ____ g ��
(5) ��װ����û������װ��B ����ʹ��������������Ԫ�ص�����������__________( �ƫС������ ƫ����Ӱ�족 )
(2) װ��A �з�Ӧ�Ļ�ѧ����ʽΪ ___ .
(3) װ��E �е������� _______________��װ��F �������� ____________________________.
(4) ��װ��C �IJ������з����������������Ϊ5.9g �������������ȼ�պ�װ��D ����7.2g ��������������к���Ԫ�ص�����Ϊ ____ g ��
(5) ��װ����û������װ��B ����ʹ��������������Ԫ�ص�����������__________( �ƫС������ ƫ����Ӱ�족 )
��1��B�е���Ӧ�����̳���
��2��2H2O2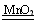 2H2O+O2����
2H2O+O2����
��3������ʯ��ˮ����ǣ���ֹ�����еĶ�����̼����E��ʹ���������ȷ��
��4��0.8��
��5��ƫ��
��2��2H2O2
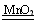 2H2O+O2����
2H2O+O2������3������ʯ��ˮ����ǣ���ֹ�����еĶ�����̼����E��ʹ���������ȷ��
��4��0.8��
��5��ƫ��

��ϰ��ϵ�д�
�����Ŀ