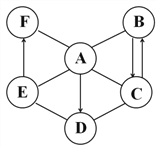
��Ŀ����
����Ŀ�����۳����ڸ��ָ߹���װ���������������Ҳ������������
I���������Ʊ�������ҵ�õ������Һ(��Ҫ��NiSO4��NiCl2)�Ʊ���ʽ̼��������xNiCO3yNi(OH)2zH2O�����������Ʊ����۵��������£�
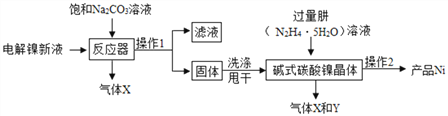
(1)��Ӧ����һ����Ҫ��ӦΪ��3NiSO4+3Na2CO3+2H2O=NiCO32Ni(OH)2��+3Na2SO4+2X��X�Ļ�ѧʽΪ____��
(2)����1������_____ , ϴ�Ӳ���1���ù���ʱ�����ô�ˮϴ�ӣ�������ϴ���ķ���(д�����衢����)___________________ ��
(3)����2�����ˡ�ˮϴ��95%�ƾ����ݡ����ɵȲ���������95%�ƾ����ݵ�Ŀ�� ______��
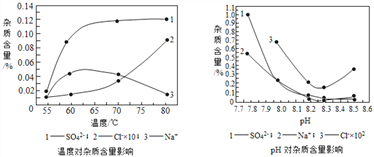
(4)�����ڷ�Ӧ���з�Ӧʱ����Ʒ�Ӧ������������ͼ����Ӧ�������ʺϵ��¶ȼ�pH�ֱ�Ϊ ________________________ ��
(5)�����У�pH�����ӣ�����Ni(OH)2��Ҳ������ӣ������ɵļ�ʽ̼���������У����ĺ�����__________������������������������������)��
���ⶨ��ʽ̼�����������ɣ�
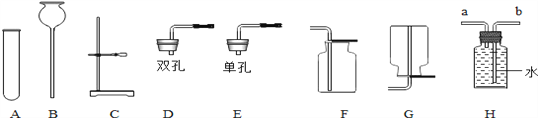
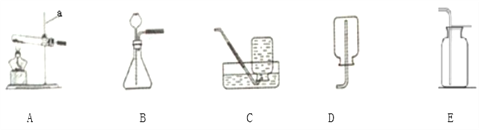
Ϊ�ⶨ��ʽ̼��������(xNiCO3yNi(OH)2zH2O��ɣ�ijС�����������ʵ�鷽����װ�ã�
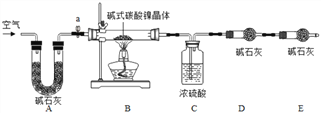
�����Ͽ�Ƭ��
(1)��ʽ̼�����������Ȼ���ȫ�ֽ�����NiO��CO2��H2O
(2)400�����ң�NiO����������Ni2O3��
��ʵ�鲽�裩��
������װ�ã�_______ ����ȷ��ȡ3.77g xNiCO3yNi(OH)2zH2O����Bװ���У������������۴��ɼ�a����������һ��ʱ�����������װ��C��D���������ܹرյ��ɼ�a������װ��B��װ��C�е���ĩ��������ð������___________����ȷ����װ��C��D���������߸������ݽ��м���(����������±�)
װ��C/g | װ��D/g | װ��E/g | |
����ǰ | 200.00 | 180.00 | 180.00 |
���Ⱥ� | 201.08 | 180.44 | 180.00 |
��ʵ����������ݴ�������
(1)����ʵ�鲽���е���գ��� ����װ�ã�_______ ����___________��
(2)װ��A�����ã� ʵ�鿪ʼǰ��ʵ�����ʱ�ֱ���__________________________
(3)����xNiCO3yNi(OH)2zH2O��x��y��z��ֵ��______________��(д���������)
���𰸡� CO2 ���� �����һ��ϴ��������Һ�м���BaCl2��Һ��Ba(NO3)2��Һ��Ba(OH) 2��Һ���۲쵽��û�г������ɡ� ʹ��������ˮ�ַ�ɢ���ƾ��У����ڹ���������ɣ� 55 8.3 ���� ���װ�������� ����ͨ����һ��ʱ�� �ž�װ����ԭ�е�CO2��ˮ���� ��װ��B�е�CO2��ˮ������ȫ�ų� x��y��z=1:2:4
��������I����1�����������غ㶨�ɷ�Ӧ��ǰ��ԭ�ӵ��������Ŀ���䣬
3NiSO4+ 3Na2CO3+ 2H2O = NiCO3��2Ni(OH)2+3Na2SO4+ 2X��
��Ӧǰ��Ni��3 ��Ӧ��Ni��3
S��3 S��3
O��23 O��19
H��4 H��4
C��3 C��1
����X��CO2
(2) ����1�������Һ��ֿ������Բ���1Ϊ���ˣ�ϴ�ӵ�Ŀ���ǽ���ʽ̼��������������������Һ�ȳ�ȥ��������ϴ�ӵ��Ƿ�ɾ���������Ƿ�����������ӣ��������һ��ϴ��������Һ�м���BaCl2��Һ��Ba(NO3)2��Һ��Ba(OH)2��Һ���۲쵽�����ɳ�����˵���Ѿ�ϴ�ɾ���(3)��95���ƾ����ݵ�Ŀ����ʹ��������ˮ�ַ�ɢ���ƾ��У����ڹ���������ɣ�(4) ��Ӧ�������ʵĺ���Խ��Խ�ã��������ʺϵ��¶�Ϊ55�����ʺϵ�pHΪ8.3��(5) ��NiCO3�����ĺ���С��Ni(OH)2�����ĺ����������У�pH�����ӣ�����Ni(OH)2����Ҳ������ӣ�NiCO3�ĺ�����Լ�С�������ɵļ�ʽ̼���������У����ĺ��������ߣ��� ��ʵ����������ݴ�������(1) ���й��������ȡ������ʵ��ǰ��Ӧ�ȼ��װ�õ������ԣ����Ⱥ�Ϊ�˽���ʽ̼�����������Ȼ���ȫ�ֽ����ɵ�CO2��H2O��ȫ��װ��C��D���գ�����ͨһ��ʱ���ȥˮ�ֺͶ�����̼�Ŀ�����(2)װ��A��������ʵ�鿪ʼǰ���ͨ������Ŀ�����ž�װ����ԭ�е�CO2��ˮ������ʵ�����ʱ���ͨ������Ŀ���ǽ�װ��B�е�CO2��ˮ������ȫ�ų� �� (3) װ��C���ӵ�������1.08g��Ϊ����ˮ��������װ��D���ӵ�������0.44g��Ϊ���ɶ�����̼������������NiO������Ϊ3.77g-1.08g-0.44g=2.25g
xNiCO3��yNi(OH)2��zH2O ![]() ��x+y��NiO + xCO2�� + ��y+z��H2O
��x+y��NiO + xCO2�� + ��y+z��H2O
75��x+y�� 44x 18��y+z��
/span> 2.25g 0.44g 1.08g
75��x+y��/ 2.25g= 44x/ 0.44g=18��y+z��/1.08g
��ã�x��y��z=1:2:4��

 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д� Сѧ�������Ծ�ϵ�д�
Сѧ�������Ծ�ϵ�д�����Ŀ������ƺ������Ƶ��ܽ�ȱ����ܽ���������¡�����˵����ȷ����
�¶�/�� | 10 | 20 | 30 | 40 | 50 | 60 | |
�ܽ�� S/g | ����� | 36.0 | 34.7 | 33.8 | 33.2 | 33.0 | 32.7 |
������ | 9.6 | 20.2 | 40.8 | 48.4 | 47.5 | 47.0 | |
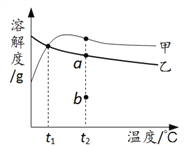
A. ��������
B. t1��Ӧ����10����20��
C. t2��ʱ����Һ��b��ת����a��ɺ�������ˮ�������
D. �ס��ҵı�����Һ��t1�����µ�t2�棬���ʵ�������������С