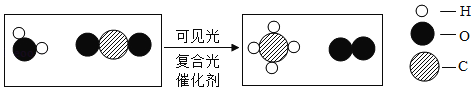
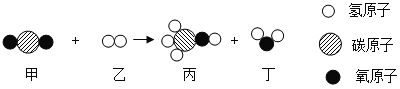
��Ŀ����
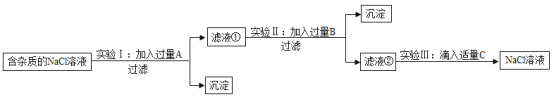
����Ŀ��Ϊ�˳�ȥNaCl��Һ�к���������MgCl2��CaCl2��Na2SO4�����ʣ�ijС��ͬѧѡ��Na2CO3��Һ��ϡ���ᡢBa��OH��2��Һ�����Լ�����һ����˳�������ͼ��ʾ��ʵ�顣
�ش��������⣺
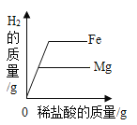
��1��ʵ�����м����Լ�A��ȥ��������_________��
��2��ʵ�����м�����Լ�B��_________��Һ���������B��Ŀ����__________��
��3����Һ���г�Na+��Cl-�⣬�����е�������__________�������ӷ��ţ���
��4��ʵ�����з������кͷ�Ӧ�Ļ�ѧ����ʽ__________��
��5��������NaCl��Һ�Ƴ��Ȼ��ƾ��壬�������Ϊ_________��
���𰸡�MgCl2��Na2SO4 Na2CO3 ��ȥ�Ȼ��ƺ������������� CO32-��OH- NaOH+HCl=NaCl+H2O �����ᾧ
��������
��1��ʵ�����м����Լ�A������������Һ����ȥ��������MgCl2��Na2SO4���Ȼ�þ��������������������þ�������Ȼ��ƣ������ƺ�����������Ӧ�������ᱵ�������Ȼ��ƣ�
��2��ʵ�����м�����Լ�B��Na2CO3��Һ���������BĿ���dz�ȥ�Ȼ��ƺ����������������Ȼ��ƺ�̼���������Ȼ��ƺ�̼��Ƴ���������������̼���Ʒ�Ӧ����̼�ᱵ�������������ƣ�
��3����Һ���г�Na+��Cl-�⣬�����е������ǹ���̼�����е�̼������ӣ�CO32-���ͷ�Ӧ�������������е����������ӣ�OH-����
��4��ʵ���������Լ�C�����ᣬ�������кͷ�Ӧ���������ƺ����ᷴӦ�����Ȼ��ƺ�ˮ����Ӧ�Ļ�ѧ����ʽ��NaOH+HCl=NaCl+H2O��
��5���Ȼ��Ƶ��ܽ�����¶�Ӱ���С��������NaCl��Һ�Ƴ��Ȼ��ƾ��壬�������Ϊ�����ᾧ��