��Ŀ����
32���ִ��������Ʒ�ʵ�Ҫ��Խ��Խ�ߣ���ʳƷ����Ҫ��ɫ���㡢ζ����Ҫ��Ӫ����ʳƷ���Ӽ�Ӧ�˶��������õ�ʳƷ���Ӽ��У�
��1����ֹʳƷ���ܱ��ʶ����ӵķ��������籽���ᣨC6H5COOH���������г��õķ���������������
��2��Ϊ����ʳƷ�й٣���������ʳ�Ρ�С�մ����ǡ�ʳ�ȣ����г��������ɼ�����
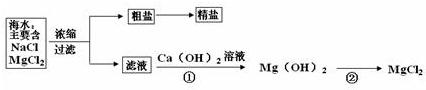
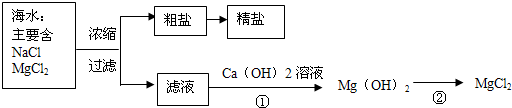
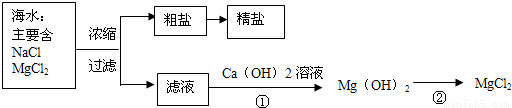
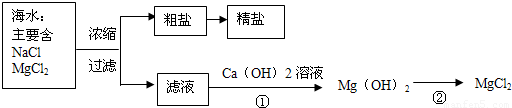
��3������±�㶹����һ�オһ���������е���±��������Ϊ�ı�ʳƷ״̬�����ӵ����̼�����±����Ҫ�ɷ����Ȼ�þ����ˮ�к��зḻ��ʳ�κ��Ȼ�þ�������ᴿ������ͼ��ʾ
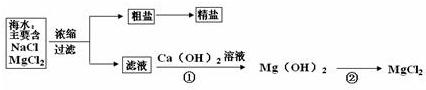
����ͼʾ�ش𣺴����к��д�����ɳ���Ӵ�����ȡ���ε�ʵ�鲽����
д���١���������Ӧ�Ļ�ѧ����ʽ
��
��
��4�����У�ͼ��ʳ���ж�������������Ӫ���������ʳ��BʳƷ������ЧԤ��
��
�����ࣻ�ڵ����ʣ�����֬�Ļ�����λ������C���Ͽ��Բ���������谱���ᣮ

��1����ֹʳƷ���ܱ��ʶ����ӵķ��������籽���ᣨC6H5COOH���������г��õķ���������������
3
��Ԫ����ɣ�����һ�������к���15
��ԭ����2��Ϊ����ʳƷ�й٣���������ʳ�Ρ�С�մ����ǡ�ʳ�ȣ����г��������ɼ�����
С�մ�
�������������������
������ͬѧ����pH��ֽ���ʳ��pHֵ������pH��ֽ��ˮ��ʪ���ÿ��ӽ�ʳ����pH��ֽ�ϣ�����õ�pHֵ����ʵ�ʽ����
�������С������ȡ�������3������±�㶹����һ�オһ���������е���±��������Ϊ�ı�ʳƷ״̬�����ӵ����̼�����±����Ҫ�ɷ����Ȼ�þ����ˮ�к��зḻ��ʳ�κ��Ȼ�þ�������ᴿ������ͼ��ʾ

����ͼʾ�ش𣺴����к��д�����ɳ���Ӵ�����ȡ���ε�ʵ�鲽����
�ܽ⡢���ˡ�����
��д���١���������Ӧ�Ļ�ѧ����ʽ
��
MgCl2+Ca��OH��2=Mg��OH��2��+CaCl2
����ܰ��ʾ�������ܽ��Ա�Ŷ��������
Mg��OH��2+2HCl=MgCl2+2H2O
����4�����У�ͼ��ʳ���ж�������������Ӫ���������ʳ��BʳƷ������ЧԤ��
���Ͳ����������ɵȣ�
��
ƶѪ
�ȼ������������������
������ţ������ࣻ�ڵ����ʣ�����֬�Ļ�����λ������C���Ͽ��Բ���������谱���ᣮ

��������1��ע��١����ʵ���ɣ�������Ԫ����ɣ��ڡ����ӵĹ��ɣ�������ԭ�ӹ��ɣ�
��2����pH��ֽ��ˮ��ʪ���൱�ڽ���ϡ�ͣ����Բ�õ�pHֵ����ʵ�ʽ��ƫ��
��3������Һ����Ҫ�����Ȼ�þ�������Ȼ�þ���������Ʒ�Ӧ�����Ȼ��ƺ�������þ����������֪��Ӧ��������þ����֪�������Ȼ�þ��������������þ�����ᷴӦ��
��4��BʳƷΪ�����߸��̷ۣ����Կ��Բ������ƣ�����������ɵ����ʵĻ�����λ��
��2����pH��ֽ��ˮ��ʪ���൱�ڽ���ϡ�ͣ����Բ�õ�pHֵ����ʵ�ʽ��ƫ��
��3������Һ����Ҫ�����Ȼ�þ�������Ȼ�þ���������Ʒ�Ӧ�����Ȼ��ƺ�������þ����������֪��Ӧ��������þ����֪�������Ȼ�þ��������������þ�����ᷴӦ��
��4��BʳƷΪ�����߸��̷ۣ����Կ��Բ������ƣ�����������ɵ����ʵĻ�����λ��
����⣺��1����������3��Ԫ����ɣ�����һ�������к���15ԭ�ӹ��ɣ�
��2�����ɼ���С�մ���ζ�������ǣ�pH��ֽ������ˮ��ʪ������Ӱ������
��3�������к��д�����ɳ���Ӵ�����ȡ���ε�ʵ�鲽�����ܽ⡢���ˡ����������ݷ�Ӧ�����������д��ѧ����ʽ��
��4��ʳ��BʳƷ������ЧԤ�������Ͳ���ƶѪ�ȣ�����������ɵ����ʣ�
�ʴ�Ϊ����1��3��15
��2��С�մ����ǡ���
��3���ܽ⡢���ˡ���������MgCl2+Ca��OH��2=Mg��OH��2��+CaCl
��Mg��OH��2+2HCl=MgCl2+2H2O
��4�����Ͳ���ƶѪ����
��2�����ɼ���С�մ���ζ�������ǣ�pH��ֽ������ˮ��ʪ������Ӱ������
��3�������к��д�����ɳ���Ӵ�����ȡ���ε�ʵ�鲽�����ܽ⡢���ˡ����������ݷ�Ӧ�����������д��ѧ����ʽ��
��4��ʳ��BʳƷ������ЧԤ�������Ͳ���ƶѪ�ȣ�����������ɵ����ʣ�
�ʴ�Ϊ����1��3��15
��2��С�մ����ǡ���
��3���ܽ⡢���ˡ���������MgCl2+Ca��OH��2=Mg��OH��2��+CaCl
��Mg��OH��2+2HCl=MgCl2+2H2O
��4�����Ͳ���ƶѪ����
������������Ҫ�����Ȼ���������ᴿ�IJ��裻������pH��ֽ������Һ������Եķ������˽������Ԫ�������Ԫ�ض����彡������Ҫ�ԣ�

��ϰ��ϵ�д�
�����Ŀ