��Ŀ����
��2008?˳����һģ���ִ��������Ʒ�ʵ�Ҫ��Խ��Խ�ߣ���ʳƷ����Ҫ��ɫ���㡢ζ����Ҫ��Ӫ����ʳƷ���Ӽ�Ӧ�˶��������õ�ʳƷ���Ӽ��У���1����ֹʳƷ���ܱ��ʶ����ӵķ��������籽���ᣨC6H5COOH���������г��õķ���������������______��Ԫ����ɣ�����һ�������к���______��ԭ�ӣ�
��2��Ϊ����ʳƷ�й٣���������ʳ�Ρ�С�մ����ǡ�ʳ�ȣ����г��������ɼ�����______����������ζ������______������ͬѧ����pH��ֽ���ʳ��pHֵ������pH��ֽ��ˮ��ʪ���ÿ��ӽ�ʳ����pH��ֽ�ϣ�����õ�pHֵ����ʵ�ʽ��______�������С������ȡ�����
��3������±�㶹����һ�オһ���������е���±��������Ϊ�ı�ʳƷ״̬�����ӵ����̼�����±����Ҫ�ɷ����Ȼ�þ����ˮ�к��зḻ��ʳ�κ��Ȼ�þ�������ᴿ������ͼ��ʾ��
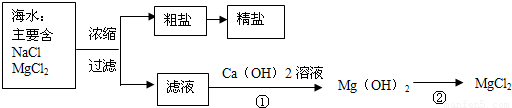
����ͼʾ�ش𣺴����к��д�����ɳ���Ӵ�����ȡ���ε�ʵ�鲽����______��
д���١���������Ӧ�Ļ�ѧ����ʽ
��______����ܰ��ʾ�������ܽ��Ա�Ŷ������
��______��
��4�����У���ͼ��ʾ��ʳ���ж�������������Ӫ���������ʳ��BʳƷ������ЧԤ��______��______�ȼ����������������______������ţ�
�����ࣻ�ڵ����ʣ�����֬�Ļ�����λ������C���Ͽ��Բ���������谱���ᣮ

���𰸡���������1�����ݱ�����Ļ�ѧʽ C6H5COOH���з������
��2��С�մ��DZ��Ƹ��ʱ�ķ��ͷۣ��ǵ���ζ����ij��Һ��PHֵ�������з������
��3�������ᴿʵ�鲽���ǣ��ܽ⡢���ˡ����������ᴿ�Ĺ��̽��з������
��4������ʳƷ��Ӫ�����ý��з������
����⣺��1�����ݱ�����Ļ�ѧʽ C6H5COOH ��֪�������� C��H��O 3����Ԫ����ɣ�
���ݱ���Ԫ�ط������½ǵ����ֱ�ʾһ������������ԭ�ӵĸ������ɴ˿�֪������һ�������к��� 15��ԭ�ӣ�
�ʴ�Ϊ��3��15��
��2������С�մ��DZ��Ƹ��ʱ�ķ��ͷۣ����������ɼ�����С�մ�������ζ������ ���ǣ����ݲ�ij��Һ��PHֵʱ������ˮʪ��PH��ֽ���ٽ�������Һ�ε�PH��ֽ�ϣ��������Һ�������ԣ�pH��ƫ����˲�õ�pHֵ����ʵ�ʽ���� �ʴ�Ϊ��С�մ����ǣ���
��3�����ݴ�����ȡ���ε�ʵ�鲽���ǣ��ܽ⡢���ˡ��������ʴ�Ϊ���ܽ⡢���ˡ�������
�ٹ��˺���Һ�����Ȼ�þ��Һ����������������Һ������������þ��������ӦʽΪ��MgCl2+Ca��OH��2=Mg��OH��2��+CaCl2��
��������м������ᣬ�����Ȼ�þ����ӦʽΪ��Mg��OH��2+2HCl=MgCl2+2H2O��
�ʴ�Ϊ��MgCl2+Ca��OH��2=Mg��OH��2��+CaCl2�� Mg��OH��2+2HCl=MgCl2+2H2O��
��4����ͼ��֪BʳƷ�Ǹ߸Ƹ����̷ۣ����ƿ���Ԥ�����Ͳ����������ɵȣ���������Ԥ��ƶѪ�����������ɶ��ְ����ṹ�ɵļ�Ϊ���ӵĻ�����ʴ�Ϊ�����Ͳ����������ɵȣ���ƶѪ�� �ڣ�
���������⿼��ѧ�����ݻ�ѧʽ�������ᴿʵ�鲽�裬��ij��Һ��PHֵ���з������⣬����֪ʶ���Ӧ�ã�
��2��С�մ��DZ��Ƹ��ʱ�ķ��ͷۣ��ǵ���ζ����ij��Һ��PHֵ�������з������
��3�������ᴿʵ�鲽���ǣ��ܽ⡢���ˡ����������ᴿ�Ĺ��̽��з������
��4������ʳƷ��Ӫ�����ý��з������
����⣺��1�����ݱ�����Ļ�ѧʽ C6H5COOH ��֪�������� C��H��O 3����Ԫ����ɣ�
���ݱ���Ԫ�ط������½ǵ����ֱ�ʾһ������������ԭ�ӵĸ������ɴ˿�֪������һ�������к��� 15��ԭ�ӣ�
�ʴ�Ϊ��3��15��
��2������С�մ��DZ��Ƹ��ʱ�ķ��ͷۣ����������ɼ�����С�մ�������ζ������ ���ǣ����ݲ�ij��Һ��PHֵʱ������ˮʪ��PH��ֽ���ٽ�������Һ�ε�PH��ֽ�ϣ��������Һ�������ԣ�pH��ƫ����˲�õ�pHֵ����ʵ�ʽ���� �ʴ�Ϊ��С�մ����ǣ���
��3�����ݴ�����ȡ���ε�ʵ�鲽���ǣ��ܽ⡢���ˡ��������ʴ�Ϊ���ܽ⡢���ˡ�������
�ٹ��˺���Һ�����Ȼ�þ��Һ����������������Һ������������þ��������ӦʽΪ��MgCl2+Ca��OH��2=Mg��OH��2��+CaCl2��
��������м������ᣬ�����Ȼ�þ����ӦʽΪ��Mg��OH��2+2HCl=MgCl2+2H2O��
�ʴ�Ϊ��MgCl2+Ca��OH��2=Mg��OH��2��+CaCl2�� Mg��OH��2+2HCl=MgCl2+2H2O��
��4����ͼ��֪BʳƷ�Ǹ߸Ƹ����̷ۣ����ƿ���Ԥ�����Ͳ����������ɵȣ���������Ԥ��ƶѪ�����������ɶ��ְ����ṹ�ɵļ�Ϊ���ӵĻ�����ʴ�Ϊ�����Ͳ����������ɵȣ���ƶѪ�� �ڣ�
���������⿼��ѧ�����ݻ�ѧʽ�������ᴿʵ�鲽�裬��ij��Һ��PHֵ���з������⣬����֪ʶ���Ӧ�ã�

��ϰ��ϵ�д�
 �Ͻ�ƽ��У����ϵ�д�
�Ͻ�ƽ��У����ϵ�д�
�����Ŀ
 ��2008?˳����һģ��������ƽ����������̳�ֽ�Ϸ�����ͭ��ĩ�����̳�ֽ�Ϸ�1��5g���룬�����ߵ�ʾ����ͼ����ʱ��ƽƽ�⣬��������ͭ������Ϊ��������
��2008?˳����һģ��������ƽ����������̳�ֽ�Ϸ�����ͭ��ĩ�����̳�ֽ�Ϸ�1��5g���룬�����ߵ�ʾ����ͼ����ʱ��ƽƽ�⣬��������ͭ������Ϊ��������