��Ŀ����
����Ŀ��ʵ����������ˮ����̼������Һ�����ᷢ���л�����������ʱ������ƣ����ú��̼������Һ���ǼӾ硣ijͬѧ�����ˡ�ϴ�ӡ����º�ɵð�ɫ���壬��������̽����
���²⣩������ˮ�к�________�������ӷ��ţ���������![]()
�ڿ����еĶ�����̼�������Һ�в��뷴Ӧ��������![]()
�۹�����![]() ��
��![]() �Ļ����
�Ļ����
�����ϣ�������ˮ���ڵͶ�Ӳˮ�����к�![]() ��
��
��![]() ���ȿɷֽ�Ϊ
���ȿɷֽ�Ϊ![]() ��
��![]() ��
��![]()
��ʵ��1����1��ȡ������ɫ���壬��ּ��Ȳ���������ʹ��ˮ����ͭ____________����²�ٲ�������
��2����ȡ8.4g��ɫ�������������ϡ���ᣬ��ַ�Ӧ���������ͨ��ʯ��ˮ���ó���������_______��ѡ������������=������������10g����²�۳�����
��ʵ��2����3��Ϊ�ⶨ������![]() ��
��![]() ��������
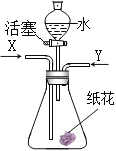
��������![]() ������ͼ��ʾװ��ʵ�顣
������ͼ��ʾװ��ʵ�顣
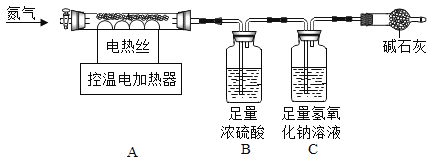
ȡһ���������ּ������������ٸı䣬��ʹ����������ȫ������ͨ����ͼװ�á��ɼ����������ݣ�
a ��Ӧǰ����������Ʒ������![]()
b ��Ӧ�������й��������Ϊ![]()
c װ��Bʵ�������![]()
d װ��Cʵ�������![]()
ijͬѧѡ��b��d��c��d����������![]() ��ֵ����
��ֵ����![]() ��
��![]() ����
����![]() ________������װ��D����ʵ��ⶨ�����________������ƫ��������ƫС��������Ӱ������������ѡ��������________��ѡ����ţ������������Ҳ�����
________������װ��D����ʵ��ⶨ�����________������ƫ��������ƫС��������Ӱ������������ѡ��������________��ѡ����ţ������������Ҳ�����![]() ��ֵ��
��ֵ��
�����죩��4���ø�����ˮ���õ�̼������Һ�����ڿ����г��ֻ��ǵ��ܷ�Ӧ����ʽ��_________��
��5�������Ͽ�֪������˵����ȷ����________
a ������![]() ���ܽ�Ƚϴ�
���ܽ�Ƚϴ�
b ʵ���Ҳ���������ˮ������������Һ
c ��ɫ���������������Һ�ж�����̼������
���𰸡�Ca2+ ���� �� 25��42 ƫ�� bc Na2CO3+CaCl2=2NaCl+CaCO3�� b
��������
{�²�}���ú��̼������Һ���ǼӾ磬���ɵĹ���ΪCaCO3�����������غ㶨�ɣ�������ˮ��һ�����и����ӣ������ΪCa2+������Ca2+��
{ʵ��1}��1���²�ٲ����������ɫ�����п϶�����̼�����ƣ�̼���������ȷ�Ӧ����̼���ơ�������̼��ˮ��ˮ����ˮ����ͭ��Ӧ������ɫ������ͭ���壬���������
��2���⣺8.4g̼��������̼Ԫ�ص�����Ϊ8.4g��![]() ��100%=1.2g��1.2g̼Ԫ���γ�̼��Ƶ�����Ϊ1.2g��
��100%=1.2g��1.2g̼Ԫ���γ�̼��Ƶ�����Ϊ1.2g��![]() ��100%=10g������²�۳�����8.4g����������CaCO3 ��NaHCO3�Ļ������������ɳ���������С��10g�������
��100%=10g������²�۳�����8.4g����������CaCO3 ��NaHCO3�Ļ������������ɳ���������С��10g�������
{ʵ��2}��3���⣺����ȡ������̼�����Ƶ�����Ϊx����Ӧ�����ɵĶ�����̼������Ϊy
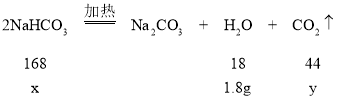
168��18=x��1.8g x=16.8g
18��44=1.8g��y y=4.4g
̼��Ʒ�Ӧ���ɶ�����̼������Ϊ8.8g4.4g=4.4g
�������̼��Ƶ�����Ϊz
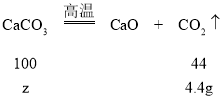
100��44=z��4.4g z=10g
������![]() ��
��![]() ��������10g��16.8g=25��42������25��42��
��������10g��16.8g=25��42������25��42��
�����װ��D��װ��C�����տ����еĶ�����̼����������̼��Ƶ�����ƫ��ʵ��ⶨ�����ƫ����ƫ��
����װ��Bʵ�������![]() ���Լ����������̼�����Ƶ���������Ӧ�������й��������Ϊ
���Լ����������̼�����Ƶ���������Ӧ�������й��������Ϊ![]() ��ȥ̼�����Ƶ��������͵õ�̼��Ƶ��������������������
��ȥ̼�����Ƶ��������͵õ�̼��Ƶ��������������������![]() ��
��![]() �������ȣ�����bc��
�������ȣ�����bc��
{����}��4���ø�����ˮ���Ƶ�̼������Һ�����ڿ����г��ֻ��ǵķ�Ӧ��̼�������Ȼ��Ʒ�Ӧ�����Ȼ��ƺ�̼��Ƴ������ʷ�Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+CaCl2=2NaCl+CaCO3������5��a ������NaHCO3���ܽ�Ƚ�С������Һ���ᾧ�γɾ��壬ѡ�����
b ����ˮ�к����Ȼ��ƣ�����ʵ���Ҳ���������ˮ������������Һ��ѡ����ȷ��
c ̼��������Һ����������еĶ�����̼��Ӧ����̼�����ƣ�����ɫ���������������Һ�ж�����̼�����й�ϵ��ѡ�������b��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�