��Ŀ����
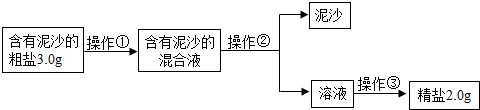
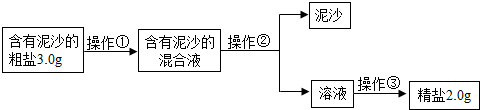
ij��ѧ��ȤС����Ƴ����д����ᴿ��ʵ�鷽��������һ��������������⣮
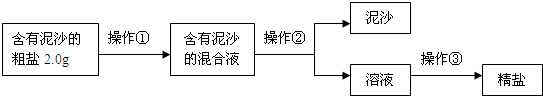
��1�������٢ڢ۵�����������
A 5mL B 8mL C 12mL
��2������������IJ���������
��3��ʵʩ������ʱ������Ҫ����
��4����ȤС���ͬѧͨ����ȷ�ļ��㷢�֣�ʵ�����þ��εIJ��ʱȸô������Ȼ��Ƶ�ʵ�ʺ���ƫ�ͣ�����ܵ�ԭ����
A������ʱ��ֽ������ B������ʱ�й��彦��
C���ܽ⺬����ɳ�Ĵ���ʱ�������ˮ������
D����������ɳ�Ļ��Һȫ���㵹�������������ˮϴ�Ӳ��������ձ�������Һ��һ�����������
E��ת�ƾ���ʱ�������������������в����Ĺ���
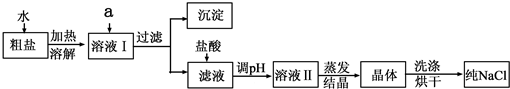
��5����ȤС���Ա�������Ϻ��֪�������г���NaCl����ɳ�⣬����������MgCl2��CaCl2��Na2SO4�����ʣ���������һ�ֽ�
�ṩ���Լ��У�Na2CO3��Һ��K2CO3��Һ��NaOH��Һ��BaCl2��Һ��Ba��NO3��2��Һ������NaCl��Һ��
������ȥ��ҺI�е�MgCl2��CaCl2��Na2SO4�����ṩ���Լ���ѡ��a���������Լ������μ�˳������Ϊ��������NaOH��Һ��������
������Һ�м������������
��6������ȤС���������ᴿ��ľ�������200g������������Ϊ0.9%��������ˮ����Ҫ���ε�����Ϊ

��1�������٢ڢ۵�����������
����
����
������
����
������
����
����ʵʩ������ǰ���Ƚ�����ĥ�飬�������������ܽⲢ��ȥ���е�����
�����ܽⲢ��ȥ���е�����
��ͬʱ�ò���������ˮ���������B
B
����֪20��ʱʳ�ε��ܽ����36g������ĸ��ţ���A 5mL B 8mL C 12mL
��2������������IJ���������
�ձ���©����������
�ձ���©����������
�����ò�������������Һ�Ի��ǣ������ԭ������ֽ����
��ֽ����
���δ�һ��ԭ���ɣ�����3��ʵʩ������ʱ������Ҫ����
����
����
���������������ֱ���ֹ���ᾧ����
�ֹ���ᾧ����
ʱֹͣ���ȣ���4����ȤС���ͬѧͨ����ȷ�ļ��㷢�֣�ʵ�����þ��εIJ��ʱȸô������Ȼ��Ƶ�ʵ�ʺ���ƫ�ͣ�����ܵ�ԭ����
BCE
BCE
������ĸ��ţ�A������ʱ��ֽ������ B������ʱ�й��彦��
C���ܽ⺬����ɳ�Ĵ���ʱ�������ˮ������
D����������ɳ�Ļ��Һȫ���㵹�������������ˮϴ�Ӳ��������ձ�������Һ��һ�����������
E��ת�ƾ���ʱ�������������������в����Ĺ���

��5����ȤС���Ա�������Ϻ��֪�������г���NaCl����ɳ�⣬����������MgCl2��CaCl2��Na2SO4�����ʣ���������һ�ֽ�
��ɳ
��ɳ
��ˮ����ķ����������һ��ʵ�鷽�����ܳ�ȥ�����е�MgCl2��CaCl2��Na2SO4�����ʣ�Ϊ�ˣ���������������˵ڶ���ʵ�鷽�����ṩ���Լ��У�Na2CO3��Һ��K2CO3��Һ��NaOH��Һ��BaCl2��Һ��Ba��NO3��2��Һ������NaCl��Һ��
������ȥ��ҺI�е�MgCl2��CaCl2��Na2SO4�����ṩ���Լ���ѡ��a���������Լ������μ�˳������Ϊ��������NaOH��Һ��������
BaCl2��Һ
BaCl2��Һ
��������Na2CO3��Һ
Na2CO3��Һ
��������Һ�м������������
��ȥ������̼���ƺ���������
��ȥ������̼���ƺ���������
����6������ȤС���������ᴿ��ľ�������200g������������Ϊ0.9%��������ˮ����Ҫ���ε�����Ϊ
1.8
1.8
g�����Ƹ���Һʱ����Ҫ��ˮӦѡ��500mL
500mL
��ѡ�50mL������100mL����500mL������Ͳ������ʱ�����������ӣ��������������������Ƶ���Һ��������������С��
��
��ѡ����ڡ�����С�ڡ����ڡ���0.9%����������1�����ݻ�������ķ���������2gȫ����ʳ���ٸ���ʳ�ε��ܽ�ȿ�����Ҫˮ��������2�����ݹ����õ����������Ժܻ��ǵ�ԭ���ǣ���3����������ʱ��ע������ǣ���4�����þ��εIJ��ʱȸô������Ȼ��Ƶ�ʵ�ʺ���ƫ��˵��ʳ�����ˣ�������һ�㶼���ԣ���5�����ݹ��˵��ص㿼�ǣ��ٸ��ݳ�ȥMgCl2��CaCl2��Na2SO4���ʣ������������Ҳ��������������ʿ��ǣ��ڸ������������̼���ƺ��������Ʒ�Ӧ���ǣ���6���������ʵļ��㷽������Ͳ��ѡȡ�������ǣ��������Ӷ�����ȡˮ�������С������
����⣺��1����ͨ�������Ѵ�Ŀ�����ȥ����ͨ�����˽�������Һ��Ĺ�����Һ����룬�����ǽ�ˮ�ֳ�ȥ�õ����Σ�������ĥ�飬�������DZ����ܽⲢ��ȥ���е����ʣ�����2gȫ����ʳ���ٸ���ʳ�ε��ܽ�ȿɼ������Ҫˮ5.56mL����������ˮ�������Ϊ8mL��
��2�������õ��������У�©�����ձ���������������̨����������IJ���������©�����ձ�������������Һ�Ի��ǣ������ԭ������ֽ�����ձ����ࡢ��Һ������ֽ��Ե��
��3������ʹ�ò��������裬��ֹ�ֲ��¶ȹ���ʹҺ�ηɽ������ֹ���������ֹͣ���ȣ������Ƚ�ʣ�����ᾧ������
��4�����þ��εIJ��ʱȸô������Ȼ��Ƶ�ʵ�ʺ���ƫ��˵��ʳ�����ˣ�������һ�㶼���ԣ�����ʱ��ֽ�������´���������Ҳ����ʳ�Σ�����ʳ���������ӣ����ʻ�ƫ�ߣ�����ʱ�й��彦���ᵼ��ʳ�μ��٣��ܽ⺬����ɳ�Ĵ���ʱ�������ˮ������ᵼ��һЩ���ʽ���ʳ�Σ�����������ƫ��������ɳ�Ļ��Һȫ���㵹�������������ˮϴ�Ӳ��������ձ�������Һ��һ�������������������ȷ������û����ת�ƾ���ʱ�������������������в����Ĺ��壬����ʳ�������٣�
��5�������dz�ȥ������Һ��Ĺ���ģ��ٳ�ȥ����MgCl2��CaCl2��Na2SO4ʵ���dz�ȥþ���ӡ������ӡ���������ӣ������dz����������ɣ���ȥþ���������������ӡ���ȥ��������̼������ӡ���ȥ����������ñ����ӣ�����Ϊ���������µ����ʣ����Լ����������ơ�̼���ơ��Ȼ������ɣ�����Ϊ̼�����dz�ȥ�Ȼ��ƺ������Ȼ����ģ�����̼���Ʊ���ŵ��Ȼ����ĺ��棻�ھ����������̼���ƺ��������Ʒ�Ӧ�����������dz�ȥ�������������ƺ�̼���Ƶģ��۾���������200g��0.9%=1.8g����ˮ200g-1.8g=198.2g����ѡ����ڸ�������ӽ��ģ���500mL��Ͳ������ʱ��������������ȡ��ˮƫ�࣬����������������ƫС��
�ʴ�Ϊ����1�����������ˣ������������ܽⲢ��ȥ���е����ʣ�B����2���ձ���©��������������ֽ���𣻣�3�����裻�ֹ���ᾧ��������4��BCE����5����ɳ����BaCl2��Һ��̼������Һ���ڳ�ȥ������̼���ƺ��������ƣ���6��1.8��500mL��С�ڣ�
��2�������õ��������У�©�����ձ���������������̨����������IJ���������©�����ձ�������������Һ�Ի��ǣ������ԭ������ֽ�����ձ����ࡢ��Һ������ֽ��Ե��
��3������ʹ�ò��������裬��ֹ�ֲ��¶ȹ���ʹҺ�ηɽ������ֹ���������ֹͣ���ȣ������Ƚ�ʣ�����ᾧ������
��4�����þ��εIJ��ʱȸô������Ȼ��Ƶ�ʵ�ʺ���ƫ��˵��ʳ�����ˣ�������һ�㶼���ԣ�����ʱ��ֽ�������´���������Ҳ����ʳ�Σ�����ʳ���������ӣ����ʻ�ƫ�ߣ�����ʱ�й��彦���ᵼ��ʳ�μ��٣��ܽ⺬����ɳ�Ĵ���ʱ�������ˮ������ᵼ��һЩ���ʽ���ʳ�Σ�����������ƫ��������ɳ�Ļ��Һȫ���㵹�������������ˮϴ�Ӳ��������ձ�������Һ��һ�������������������ȷ������û����ת�ƾ���ʱ�������������������в����Ĺ��壬����ʳ�������٣�
��5�������dz�ȥ������Һ��Ĺ���ģ��ٳ�ȥ����MgCl2��CaCl2��Na2SO4ʵ���dz�ȥþ���ӡ������ӡ���������ӣ������dz����������ɣ���ȥþ���������������ӡ���ȥ��������̼������ӡ���ȥ����������ñ����ӣ�����Ϊ���������µ����ʣ����Լ����������ơ�̼���ơ��Ȼ������ɣ�����Ϊ̼�����dz�ȥ�Ȼ��ƺ������Ȼ����ģ�����̼���Ʊ���ŵ��Ȼ����ĺ��棻�ھ����������̼���ƺ��������Ʒ�Ӧ�����������dz�ȥ�������������ƺ�̼���Ƶģ��۾���������200g��0.9%=1.8g����ˮ200g-1.8g=198.2g����ѡ����ڸ�������ӽ��ģ���500mL��Ͳ������ʱ��������������ȡ��ˮƫ�࣬����������������ƫС��
�ʴ�Ϊ����1�����������ˣ������������ܽⲢ��ȥ���е����ʣ�B����2���ձ���©��������������ֽ���𣻣�3�����裻�ֹ���ᾧ��������4��BCE����5����ɳ����BaCl2��Һ��̼������Һ���ڳ�ȥ������̼���ƺ��������ƣ���6��1.8��500mL��С�ڣ�
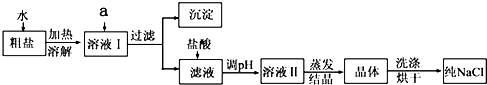
������ͨ���ش���֪���˻�������ķ����ͳ�ȥ���������ʵķ��������������µ����ʣ�����ע��˳��

��ϰ��ϵ�д�
 ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д� ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�
�����Ŀ