��Ŀ����
ij��ѧ��ȤС����Ƴ����д����ᴿ��ʵ�鷽������ش��������⣮
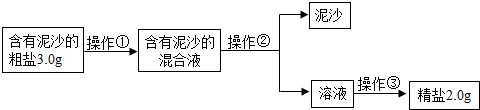
��1����ʵʩ������ǰ���Ƚ�����ĥ�飬��������
��2������������IJ���������
��3��ʵʩ������ʱ������Ҫ���Ͻ���ֱ��
��4����ȤС���ͬѧͨ����ȷ�ļ��㷢�֣�ʵ�����þ��εIJ��ʱȸô������Ȼ��Ƶ�ʵ�ʺ���ƫ�ͣ�����ܵ�ԭ����
A������ʱ��ֽ�����𡡡�����������B������ʱ�й��彦��
C���ܽ⺬����ɳ�Ĵ���ʱ�������ˮ������
D����������ɳ�Ļ��Һȫ���㵹�������������ˮϴ�Ӳ��������ձ�������Һ��һ�����������
E��ת�ƾ���ʱ�������������������в����Ĺ���
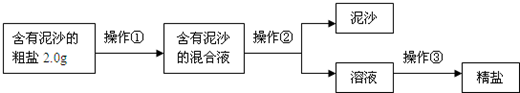
��5����ȤС���Ա�������Ϻ��֪�������г���NaCl����ɳ�⣬����������MgCl2��CaCl2��Na2SO4�ȿ��������ʣ������˲������ܳ�ȥ�����еĿ��������ʣ�Ϊ����������������˵ڶ���ʵ�鷽����
�ṩ���Լ��У�Na2CO3��Һ��K2CO3��Һ��NaOH��Һ��BaCl2��Һ��Ba��NO3��2��Һ������NaCl��Һ��
������ȥ��ҺI�е�MgCl2��CaCl2��Na2SO4�����ṩ���Լ���ѡ��a���������Լ������μ�˳������Ϊ��������NaOH��Һ��������
������Һ�м������������
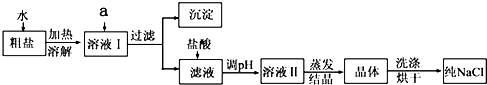
��6������ȤС���������ᴿ��ľ�������200g������������Ϊ0.9%��������ˮ����ȡˮʱѡ�����Ͳ�����

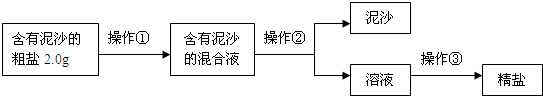
��1����ʵʩ������ǰ���Ƚ�����ĥ�飬��������
�����ܽⲢ��ȥ���е�����
�����ܽⲢ��ȥ���е�����
����2������������IJ���������
�ձ���©����������
�ձ���©����������
�����ò�������������Һ�Ի��ǣ������ԭ������ֽ����
��ֽ����
���δ�һ��ԭ�ɣ�����3��ʵʩ������ʱ������Ҫ���Ͻ���ֱ��
�н϶��������
�н϶��������
ʱֹͣ���ȣ���4����ȤС���ͬѧͨ����ȷ�ļ��㷢�֣�ʵ�����þ��εIJ��ʱȸô������Ȼ��Ƶ�ʵ�ʺ���ƫ�ͣ�����ܵ�ԭ����
BCE
BCE
������ĸ��ţ�A������ʱ��ֽ�����𡡡�����������B������ʱ�й��彦��
C���ܽ⺬����ɳ�Ĵ���ʱ�������ˮ������
D����������ɳ�Ļ��Һȫ���㵹�������������ˮϴ�Ӳ��������ձ�������Һ��һ�����������
E��ת�ƾ���ʱ�������������������в����Ĺ���
��5����ȤС���Ա�������Ϻ��֪�������г���NaCl����ɳ�⣬����������MgCl2��CaCl2��Na2SO4�ȿ��������ʣ������˲������ܳ�ȥ�����еĿ��������ʣ�Ϊ����������������˵ڶ���ʵ�鷽����
�ṩ���Լ��У�Na2CO3��Һ��K2CO3��Һ��NaOH��Һ��BaCl2��Һ��Ba��NO3��2��Һ������NaCl��Һ��

������ȥ��ҺI�е�MgCl2��CaCl2��Na2SO4�����ṩ���Լ���ѡ��a���������Լ������μ�˳������Ϊ��������NaOH��Һ��������
BaCl2��Һ
BaCl2��Һ
��������Na2CO3��Һ
Na2CO3��Һ
��������Һ�м������������
��ȥ������̼���ƺ���������
��ȥ������̼���ƺ���������
����6������ȤС���������ᴿ��ľ�������200g������������Ϊ0.9%��������ˮ����ȡˮʱѡ�����Ͳ�����
500mL
500mL
��ѡ�50mL������100mL����500mL����������ʱ�����������ӣ�����������������������Һ��������������С��
��
0.9%��ѡ����ڡ�����С�ڡ����ڡ�������������1���Ѵ�����ϸ�����Լӿ���ε��ܽ����ʣ�
��2��������Ҫ�IJ����������ձ���©��������������ֽ����Һ��߳���ֽ��Ե���н���Һ���ձ����ྻ�����Ե�����Һ��Ȼ���ǣ�
��3������ʱ�������ֽ϶����ʱҪֹͣ���ȣ�
��4������ʱ�й��彦�����ᵼ���Ȼ��Ƶ���ʧ���ܽ⺬����ɳ�Ĵ���ʱ�������ˮ�����㣬�ᵼ�´����е��Ȼ��Ʋ�����ȫ�ܽ⣬ת�ƾ���ʱ�������������������в����Ĺ��壬�ᵼ���Ȼ��Ƶ���ʧ����Щ�����Ե������þ��εIJ��ʱȸô������Ȼ��Ƶ�ʵ�ʺ���ƫ�ͣ�
��5���ټ���������������ƿ��Գ�ȥ�Ȼ�þ������������Ȼ������Գ�ȥ�����ƣ����������̼���ƿ��Գ�ȥ�Ȼ��ƺ������Ȼ�����
������Һ�м�������ѹ������������ƺ�̼���Ƴ�ȥ��
��6��ѡȡ��Ͳʱ����Ͳ�Ĺ��Ӧ�ô��ڻ����Ҫ��ȡ��Һ�����������Һ���ȡ�������ӽ���
��2��������Ҫ�IJ����������ձ���©��������������ֽ����Һ��߳���ֽ��Ե���н���Һ���ձ����ྻ�����Ե�����Һ��Ȼ���ǣ�
��3������ʱ�������ֽ϶����ʱҪֹͣ���ȣ�
��4������ʱ�й��彦�����ᵼ���Ȼ��Ƶ���ʧ���ܽ⺬����ɳ�Ĵ���ʱ�������ˮ�����㣬�ᵼ�´����е��Ȼ��Ʋ�����ȫ�ܽ⣬ת�ƾ���ʱ�������������������в����Ĺ��壬�ᵼ���Ȼ��Ƶ���ʧ����Щ�����Ե������þ��εIJ��ʱȸô������Ȼ��Ƶ�ʵ�ʺ���ƫ�ͣ�
��5���ټ���������������ƿ��Գ�ȥ�Ȼ�þ������������Ȼ������Գ�ȥ�����ƣ����������̼���ƿ��Գ�ȥ�Ȼ��ƺ������Ȼ�����
������Һ�м�������ѹ������������ƺ�̼���Ƴ�ȥ��
��6��ѡȡ��Ͳʱ����Ͳ�Ĺ��Ӧ�ô��ڻ����Ҫ��ȡ��Һ�����������Һ���ȡ�������ӽ���
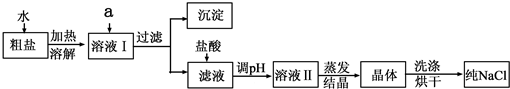
����⣺��1�����ܽ�ǰ���Ƚ�����ĥ�飬�������DZ����ܽⲢ��ȥ���е����ʣ�
��������ܽⲢ��ȥ���е����ʣ�
��2��������Ҫ�IJ����������ձ���©��������������ֽ����Һ��߳���ֽ��Ե���н���Һ���ձ����ྻ�����Ե�����Һ��Ȼ���ǣ�
����ձ���©��������������ֽ����
��3������ʱ������Ҫ���Ͻ���ֱ���н϶��������ʱֹͣ���ȣ�
����н϶����������
��4������ʱ�й��彦�����ᵼ���Ȼ��Ƶ���ʧ���ܽ⺬����ɳ�Ĵ���ʱ�������ˮ�����㣬�ᵼ�´����е��Ȼ��Ʋ�����ȫ�ܽ⣬ת�ƾ���ʱ�������������������в����Ĺ��壬�ᵼ���Ȼ��Ƶ���ʧ����Щ�����Ե������þ��εIJ��ʱȸô������Ȼ��Ƶ�ʵ�ʺ���ƫ�ͣ�
���BCE��
��5���ټ���������������ƿ��Գ�ȥ�Ȼ�þ������������Ȼ������Գ�ȥ�����ƣ����������̼���ƿ��Գ�ȥ�Ȼ��ƺ������Ȼ�����
���BaCl2��Һ��Na2CO3��Һ��
������Һ�м�����������dz�ȥ������̼���ƺ��������ƣ�
�����ȥ������̼���ƺ��������ƣ�
��6������200g������������Ϊ0.9%��������ˮʱ����Ҫˮ������Ϊ��200g-200g��0.9%=198.2g��ˮ�������198.2mL��Ӧ����500mL����Ͳ��
����������ʱ����ȡ��ˮ���������198.2mL������������Һ��������������С��0.9%��
���500mL�� С�ڣ�
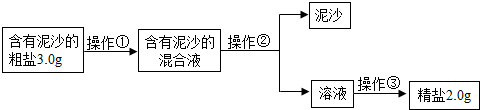
��������ܽⲢ��ȥ���е����ʣ�
��2��������Ҫ�IJ����������ձ���©��������������ֽ����Һ��߳���ֽ��Ե���н���Һ���ձ����ྻ�����Ե�����Һ��Ȼ���ǣ�
����ձ���©��������������ֽ����
��3������ʱ������Ҫ���Ͻ���ֱ���н϶��������ʱֹͣ���ȣ�
����н϶����������
��4������ʱ�й��彦�����ᵼ���Ȼ��Ƶ���ʧ���ܽ⺬����ɳ�Ĵ���ʱ�������ˮ�����㣬�ᵼ�´����е��Ȼ��Ʋ�����ȫ�ܽ⣬ת�ƾ���ʱ�������������������в����Ĺ��壬�ᵼ���Ȼ��Ƶ���ʧ����Щ�����Ե������þ��εIJ��ʱȸô������Ȼ��Ƶ�ʵ�ʺ���ƫ�ͣ�
���BCE��
��5���ټ���������������ƿ��Գ�ȥ�Ȼ�þ������������Ȼ������Գ�ȥ�����ƣ����������̼���ƿ��Գ�ȥ�Ȼ��ƺ������Ȼ�����
���BaCl2��Һ��Na2CO3��Һ��
������Һ�м�����������dz�ȥ������̼���ƺ��������ƣ�
�����ȥ������̼���ƺ��������ƣ�
��6������200g������������Ϊ0.9%��������ˮʱ����Ҫˮ������Ϊ��200g-200g��0.9%=198.2g��ˮ�������198.2mL��Ӧ����500mL����Ͳ��
����������ʱ����ȡ��ˮ���������198.2mL������������Һ��������������С��0.9%��
���500mL�� С�ڣ�
��������ѧʵ�������ǻ�ѧʵ����ͻ�����������IJ��֣�Ҳ�ǽ��з��������ó����۵����ݣ��������ʵ����ʺ��֮��ķ�Ӧ��ϵ����������߹۲�ʵ�顢����ʵ������������ԣ��Ի�ѧʵ�鲻��Ҫ����۲죬��Ӧ�������ʵ�顢�۲�ʵ������ķ�����

��ϰ��ϵ�д�
�����Ŀ