��Ŀ����
��һ��ʯ��ʯ��Ʒ�ijɷ���CaCO3��SiO2������С��ͬѧ��100g�����5�μ��뵽35gʯ��ʯ��Ʒ�У���֪SiO2�������ᷴӦ�����õ����²������ݺ�ͼ��| ���� | ��1�� | ��2�� | ��3�� |
| �������������/g | 20 | 20 | 20 |
| ʣ����������/g | 30 | a | 20 |
��1����2�μ��������aΪ______g��
��2��ʯ��ʯ��Ʒ�и�Ԫ�ء�̼Ԫ�غ���Ԫ�ص�������Ϊ���٣����������������ȱ�ʾ��
��3��10%��CaCl2��Һ����·�汣ʪ����������5��ʵ������Һ���10%��CaCl2��Һ�����������Һ�м���������ʯ��ʯ��ĩ����ȫ��Ӧ����ˣ���ʱ����Ҫ����Һ�м���ˮ���ٿˣ�������ʵ���������Һ��ʧ���Բ��ƣ�
���𰸡���������1����ͼ�����������βμӷ�Ӧ������������ȣ��ɴ˼�����ڶ��βμӷ�Ӧ����������������ʣ����������=������-�μӷ�Ӧ�����������ɼ����a��ֵ��
��2�����������ͼ���������ɵõ���Ʒ��CaCO3���������ٸ���CaCO3�и�Ԫ�ء�̼Ԫ�غ���Ԫ�ص�������������Ǹ�ռ�ı�ֵ��
��3���ɻ�ѧ����ʽ���Լ������100gϡ���ᷴӦ��̼��Ƶ�������ͬʱ���ܼ�������ɵ��Ȼ����Լ�������̼���������ٸ��������غ㶨�ɿ��Լ������Ӧ���Ȼ�����Һ��������������ϡ��ǰ�����ʵ��������伴�ɼ�����Ҫ����Һ�м���ˮ��������
����⣺��1����ͼ���֪�����Ĵμ�������ŵõ���ȫ��Ӧ�����ڷ�Ӧ�����У���һ�γ�ַ�Ӧ��ʣ����������Ϊ30g��˵��ֻ��35g-30g=5g��Ʒ�����˷�Ӧ�������γ�ַ�Ӧ��ʣ����������Ϊ20g��˵������35g-20g=15g��Ʒ�����˷�Ӧ���ʵڶ��γ�ַ�Ӧʱ��Ҳֻ��5g��Ʒ�����˷�Ӧ���ʵ�2�μ��������a=35g-5g×2=25g��
�ʴ�Ϊ25��
��2������ͼ���������4�μ����������Ʒ��CaCO3��ȫ��Ӧ��
��Ʒ�и�Ԫ�ء�̼Ԫ�غ���Ԫ�ص������ȣ�
��20g× ������20g×
������20g× ������20g×
������20g×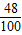 +15g×
+15g×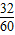 ��=10��3��22
��=10��3��22
��ʯ��ʯ��Ʒ�и�Ԫ�ء�̼Ԫ�غ���Ԫ�ص�������Ϊ10��3��22��
��3����ͼ����֪��ÿ����20g���ᣬ����5gCaCO3����100g��������25gCaCO3��
�裺100g������ȫ��Ӧ����Һ��CaCl2������Ϊx������CO2������Ϊy��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 111 44
25g x y
 =
= ��
��
 =
= ��
��
��ã�x=27.75g��y=11g��
����CaCl2��Һ��������25g+100g-11g=114g
�軹�����ˮ������Ϊz��
�� ×100%=10%��
×100%=10%��
��ã�z=163.5g��
�𣺻�������Һ�м�ˮ163.5g��
����������Ҫ��ѧ���˽���ȫ��Ӧ�Ͳ���ȫ��Ӧ�ĸ���ص㣬�۲�ͼ�����������ݣ���ȷ����ڼ���ʯ��ʯ��Ʒ�и�Ԫ�ء�̼Ԫ�غ���Ԫ�ص�������Ϊʱ��һ��ע����Ԫ�ز�ֻ̼������У�����������Ҳ����Ԫ�أ�
��2�����������ͼ���������ɵõ���Ʒ��CaCO3���������ٸ���CaCO3�и�Ԫ�ء�̼Ԫ�غ���Ԫ�ص�������������Ǹ�ռ�ı�ֵ��
��3���ɻ�ѧ����ʽ���Լ������100gϡ���ᷴӦ��̼��Ƶ�������ͬʱ���ܼ�������ɵ��Ȼ����Լ�������̼���������ٸ��������غ㶨�ɿ��Լ������Ӧ���Ȼ�����Һ��������������ϡ��ǰ�����ʵ��������伴�ɼ�����Ҫ����Һ�м���ˮ��������
����⣺��1����ͼ���֪�����Ĵμ�������ŵõ���ȫ��Ӧ�����ڷ�Ӧ�����У���һ�γ�ַ�Ӧ��ʣ����������Ϊ30g��˵��ֻ��35g-30g=5g��Ʒ�����˷�Ӧ�������γ�ַ�Ӧ��ʣ����������Ϊ20g��˵������35g-20g=15g��Ʒ�����˷�Ӧ���ʵڶ��γ�ַ�Ӧʱ��Ҳֻ��5g��Ʒ�����˷�Ӧ���ʵ�2�μ��������a=35g-5g×2=25g��
�ʴ�Ϊ25��
��2������ͼ���������4�μ����������Ʒ��CaCO3��ȫ��Ӧ��
��Ʒ�и�Ԫ�ء�̼Ԫ�غ���Ԫ�ص������ȣ�
��20g×
 ������20g×
������20g× ������20g×
������20g×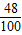 +15g×
+15g× ��=10��3��22
��=10��3��22��ʯ��ʯ��Ʒ�и�Ԫ�ء�̼Ԫ�غ���Ԫ�ص�������Ϊ10��3��22��
��3����ͼ����֪��ÿ����20g���ᣬ����5gCaCO3����100g��������25gCaCO3��
�裺100g������ȫ��Ӧ����Һ��CaCl2������Ϊx������CO2������Ϊy��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 111 44
25g x y
 =
= ��
�� =
= ��
����ã�x=27.75g��y=11g��
����CaCl2��Һ��������25g+100g-11g=114g
�軹�����ˮ������Ϊz��
��
 ×100%=10%��
×100%=10%����ã�z=163.5g��
�𣺻�������Һ�м�ˮ163.5g��
����������Ҫ��ѧ���˽���ȫ��Ӧ�Ͳ���ȫ��Ӧ�ĸ���ص㣬�۲�ͼ�����������ݣ���ȷ����ڼ���ʯ��ʯ��Ʒ�и�Ԫ�ء�̼Ԫ�غ���Ԫ�ص�������Ϊʱ��һ��ע����Ԫ�ز�ֻ̼������У�����������Ҳ����Ԫ�أ�

��ϰ��ϵ�д�
�����Ŀ
 ��һ��ʯ��ʯ��Ʒ����Ҫ�ɷ���CaCO3������С��ͬѧ��100gϡ�����5�μ��뵽35gʯ��ʯ��Ʒ�У���֪���ʲ������ᷴӦҲ������ˮ�����õ����²������ݺ�ͼ������ϸ�۲����б����ͼ����㣺
��һ��ʯ��ʯ��Ʒ����Ҫ�ɷ���CaCO3������С��ͬѧ��100gϡ�����5�μ��뵽35gʯ��ʯ��Ʒ�У���֪���ʲ������ᷴӦҲ������ˮ�����õ����²������ݺ�ͼ������ϸ�۲����б����ͼ����㣺| ���� | ��1�� | ��2�� | ��3�� |
| �������������/g | 20 | 20 | 20 |
| ʣ����������/g | 30 | a | 20 |
��2��ʯ��ʯ��Ʒ��CaCO3��������Ϊ���٣��������ȷ��0.1%��
��3��10%��CaCl2��Һ����Ϊ·�汣ʪ����������5��ʵ������Һ���10%��CaCl2��Һ�����������Һ�м���������ʯ���ҷ�ĩ����ȫ��Ӧ����ˣ���ʱ����Ҫ����Һ�м���ˮ���ٿˣ�������ʵ���������Һ��ʧ���Բ��ƣ�
 ��һ��ʯ��ʯ��Ʒ�ijɷ���CaCO3��SiO2������С��ͬѧ��100g�����5�μ��뵽35gʯ��ʯ��Ʒ�У���֪SiO2�������ᷴӦ�����õ����²������ݺ�ͼ��
��һ��ʯ��ʯ��Ʒ�ijɷ���CaCO3��SiO2������С��ͬѧ��100g�����5�μ��뵽35gʯ��ʯ��Ʒ�У���֪SiO2�������ᷴӦ�����õ����²������ݺ�ͼ��| ���� | ��1�� | ��2�� | ��3�� |
| �������������/g | 20 | 20 | 20 |
| ʣ����������/g | 30 | a | 20 |
��1����2�μ��������aΪ
��2��ʯ��ʯ��Ʒ�и�Ԫ�ء�̼Ԫ�غ���Ԫ�ص�������Ϊ���٣����������������ȱ�ʾ��
��3��10%��CaCl2��Һ����·�汣ʪ����������5��ʵ������Һ���10%��CaCl2��Һ�����������Һ�м���������ʯ��ʯ��ĩ����ȫ��Ӧ����ˣ���ʱ����Ҫ����Һ�м���ˮ���ٿˣ�������ʵ���������Һ��ʧ���Բ��ƣ�
��һ��ʯ��ʯ��Ʒ�ijɷ���CaCO3��SiO2������С��ͬѧ��100g�����5�μ��뵽35gʯ��ʯ��Ʒ�У���֪SiO2�������ᷴӦ�����õ����²������ݣ�
������5��ʵ������Һ���10%��CaCl2��Һ�����������Һ�м���������ʯ��ʯ��ĩ����ȫ��Ӧ����ˣ�����㣺��ʱ����Ҫ����Һ�м���ˮ���ٿˣ�������ʵ���������Һ��ʧ���Բ��ƣ�
| ���� | ��1�� | ��2�� | ��3�� | ��4�� | ��5�� |
| �������������/g | 20 | 20 | 20 | 20 | 20 |
| ʣ����������/g | 30 | 25 | 20 | 15 | 15 |
��һ��ʯ��ʯ��Ʒ�ijɷ���CaCO3��SiO2������С��ͬѧ��100g�����5�μ��뵽35gʯ��ʯ��Ʒ�У���֪SiO2������ˮҲ�������ᷴӦ�����õ����±��������ݣ�
����㣺
��1����2�μ��������aΪ g��
��2��ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ���٣�
��3��10%��CaCl2��Һ����·�汣ʪ����������5��ʵ������Һ���10%��CaCl2��Һ�����������Һ�м���������ʯ��ʯ��ĩ����ȫ��Ӧ����ˣ���ʱ����Ҫ����Һ�м���ˮ���ٿˣ�������ʵ���������Һ��ʧ���Բ��ƣ�
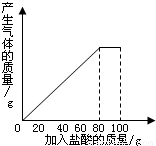
��4������35gʯ��ʯ��Ʒ�м��������������ʣ�����������仯��ϵ��ʾ��ͼ���ڡ��������ͼ����ͼ��
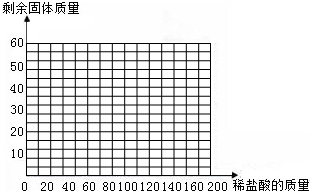
| ���� | ��1�� | ��2�� | ��3�� | ��4�� | ��5�� |
| �������������/g | 20 | 20 | 20 | 20 | 20 |
| ʣ����������/g | 30 | a | 20 | 15 | 15 |
��1����2�μ��������aΪ
��2��ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ���٣�
��3��10%��CaCl2��Һ����·�汣ʪ����������5��ʵ������Һ���10%��CaCl2��Һ�����������Һ�м���������ʯ��ʯ��ĩ����ȫ��Ӧ����ˣ���ʱ����Ҫ����Һ�м���ˮ���ٿˣ�������ʵ���������Һ��ʧ���Բ��ƣ�
��4������35gʯ��ʯ��Ʒ�м��������������ʣ�����������仯��ϵ��ʾ��ͼ���ڡ��������ͼ����ͼ��
